Gemological Characteristics of Coated Jadeite Jade

ABSTRACT
Ten coated jadeite jade samples were investigated by standard gemological testing, DiamondView imaging, confocal laser scanning microscopy, and spectrophotometry, including visible-range, FTIR, and Raman. The light green coating’s Mohs hardness and refractive index were much lower than that of uncoated jadeite. Portions of the coating had peeled off in some cases, exposing the true color of the jadeite below. Measurements using a confocal scanning microscope indicated an average coating thickness of about 16 μm. Diamond-View observations revealed a difference in fluorescence reaction between the surface coating and the jadeite beneath it. Visible-range spectroscopy showed a weaker broad band at approximately 630 nm and a moderate peak at 437 nm, without the approximately 691 nm band found in untreated jadeite containing chromium. The coated samples’ FTIR spectra contained four characteristic bands at about 2856, 2873, 2928, and 2958 cm–1, while the Raman spectra showed a broad band near 2000 cm–1. These results indicated the presence of an organic polymer in the coating material.
INTRODUCTION
Jadeite jade can be coated with a thin organic layer to improve color and transparency, and to conceal internal or external features (Koivula et al., 1994; Zhang, 2006). The attractive luster of coated jadeite can be quite deceptive, as shown in figure 1. After emerging in the early 1980s, the material enjoyed great popularity in the mid-1980s, before gradually diminishing (Ouyang, 2000; Zhang, 2006). Since 2011, with the industry facing a serious shortage of rough jadeite due to the political and economic instability in Myanmar, the coating of low-quality jadeite has reemerged in the Chinese markets, especially in Guangdong and Yunnan provinces (Liang, 2012), where the important jadeite dealers and manufacturers are located.
The early form of the coating was applied mostly to jadeite bangles and cabochons. Because of surface tension, epoxy resin often cools to form a natural curved shape, which would not significantly alter the appearance of these items. With developments in coating technology, dry-color (low luster and transparency) jadeite carvings such as kosmochlor and hte long sein have also been coated to add luster. Both have an attractive green color but poor transparency (Cui et al., 1999; Ouyang and Qi, 2001). The new coating is better at removing air bubbles between the coating and the jade, and it adheres more firmly to the gem material. It also enhances color and transparency and is more difficult to detect. With advances in coating technology, the treatment has been applied not only to jadeite, but also to other gems such as amber and pearl (Li et al., 2011; Hyatt, 2012).
Although Koivula et al. (1994) reported on the major identifying characteristics of coated jadeite, such as microscopic features and the difference in RI between the coated layer and the material beneath it, a detailed study is warranted. This paper presents an investigation of the coating material using advanced analytical techniques.

MATERIALS AND METHODS
Ten coated green jadeite cabochons ranging from 1.43 to 2.71 ct (figure 1) were obtained from the Guangzhou gem market in China’s Guangdong province. In three of the samples, part of the coating had peeled off, exposing a light green transparent coating and light green jadeite underneath. In one sample, we intentionally removed the coating for this study. Figure 2 shows this sample before and after the coating was removed. For comparison, we also examined five uncoated green jadeite cabochons between 4.17 and 6.07 ct (figure 3) from the National Gemstone Testing Center in Beijing. Hardness of both the coating and the jadeite was tested.

The samples were observed with a standard binocular microscope using various lighting techniques (reflected light, fiber-optic, and darkfield illumination) and magnification up to 20×. UV fluorescence reaction was observed in a dark room with a conventional 4-watt combination long-wave (365 nm) and short-wave (254 nm) UV lamp. The coated samples were also examined with a De Beers DTC DiamondView deep-UV (less than 230 nm) luminescence imaging system (Zhang et al., 2012). All samples were observed under a Chelsea color filter. Refractive indices were measured with a standard refractometer, and specific gravity was determined hydrostatically.
The thickness of the coating was measured with a confocal laser scanning microscope (Olympus LEXT OLS4000 3D) using brightfield illumination. The microscope has five key laser technologies, three observation modes, and seven measurement modes, so it can offer high resolution, accurate depth positioning, dynamic observation, and longitudinal scanning (Knebel and Ulrich, 2002), making it suitable for measurement of the coating. We used the step measurement mode, which allows measurement between any two arbitrary points on a surface profile with a scale resolution up to 10 nm. The accuracy of the thickness measurement depends on the magnification and software used. Normally, the accuracy is about ±2%. When measuring the thickness of the coating in the measurement window of the LEXT OLS4000 software, we followed these steps: First, we adjusted the focal point of the microscope onto the surface of the coating and selected the desired measurement position. Next, we selected the vertical measurement mode and dragged a line from the position to the uncoated area, which allowed the machine to focus on two different surfaces and determine the depth in between.
A variety of spectroscopic techniques were used to analyze the samples. For 10 coated and four uncoated green jadeites, we collected absorption spectra in the visible-range (400–700 nm) at room temperature using a Hitachi UV-3300 spectrophotometer with a sampling interval of 0.1 nm and a spectral bandwidth of 1 nm. Absorption spectra in the mid-IR region (4000–400 cm−1; 1 cm−1 resolution) were recorded for 10 coated and five uncoated samples at room temperature with a Thermo-Nicolet Nexus 670 Fourier-transform infrared (FTIR) spectrometer. A total of 128 scans per spectrum were collected to improve the signal-to-noise ratio. Raman spectra were recorded on four of the coated samples using a Renishaw Raman-1000 microspectrometer with an Ar-ion laser operating at 514.5 nm excitation between 100 and 4000 cm−1. Raman spectra were collected at room temperature, and up to three scans were accumulated to achieve a better signal-to-noise ratio.

RESULTS AND DISCUSSION
Gemological Features. Coated jadeite feels dry and rough to the touch. Due to the coating’s poor thermal conductivity, the material also feels warm, unlike the smooth, cold surface of uncoated jadeite. The Mohs hardness of the coating was approximately 3, much lower than that of uncoated jadeite (about 7). Observed under magnification, the coated jadeite often displayed small traces of scratching. These irregular scratches were different from the polishing lines on uncoated jadeite. The green color was concentrated in the surface layer, while the material below had a less attractive color. In addition, gaps could be found between the coating and the jadeite, as shown in figure 4.

Using a laser scanning confocal microscope, the observer can clearly distinguish between the coated and uncoated areas, while also measuring the thickness of the coating. Figure 6, magnified 240×, clearly demonstrates these irregular surface scratches. The measured thickness of the coating was 15.982 µm.

The coated samples showed consistent fluorescence reactions to short- and long-wave UV radiation. When exposed to long-wave UV radiation, the coating exhibited a weak slightly blue reaction while the jadeite below gave a slightly yellowish green reaction of weak to moderate intensity. Exposure to short-wave UV produced weaker reactions of the same color.
The samples displayed a variety of DiamondView reactions. The coating typically fluoresced slightly blue with alternating brighter spots, while the uncoated areas exhibited a weak to strong yellowish green fluorescence (figure 7).
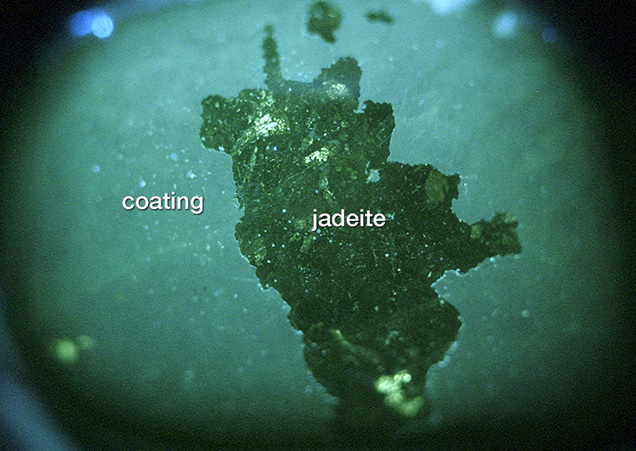
jadeite sample. Photo by Jian Zhang.
Testing of the 10 coated samples showed that the coated areas had an average RI of about 1.53, measured by the distant vision method, while the uncoated areas averaged 1.66. The color did not change under the Chelsea color filter. The average SG of the coated samples was 3.29, essentially indistinguishable from that of uncoated jadeite.
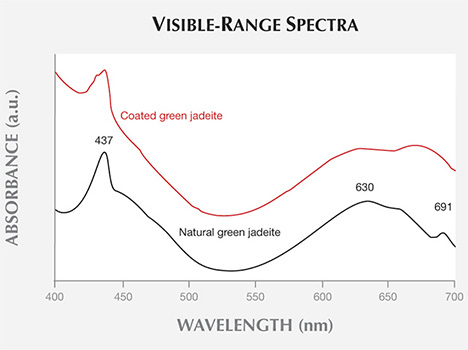
Spectroscopic Features. Viewing the coated samples with a standard desk-model spectroscope, we typically observed a weak to moderate band at 437 nm and a weaker broad band at approximately 630 nm, without the 691 nm peak found in uncoated jadeite containing chromium. The 437 nm absorption band caused by a trivalent (ferric) ion is common in jadeite of various colors (Koivula et al., 1994; Yuan et al., 2003). The broad band centered at 630 nm and the 691 nm band are induced by trivalent chromium, the most important color-causing element in untreated green jadeite (Yuan et al., 2003). Yet the absorption of the weaker broad band at approximately 630 nm in the coated samples may have been caused by the chromium in the coating (Ouyang, 2000). The visible-range spectra of the coated and uncoated green jadeite can be seen in figure 8.
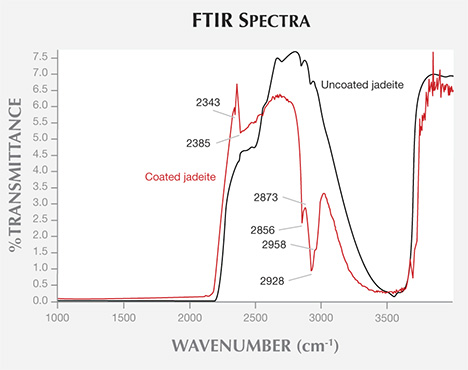
The FTIR spectra of the coated jadeites revealed two distinct bands at 2343 and 2385 cm−1, as well as four characteristic peaks at 2856, 2873, 2928, and 2958 cm−1. The two distinct bands at 2343 and 2385 cm−1, which are not found in the absorption spectra of uncoated jadeite, may be related to carbon dioxide at room temperature (Ning, 2010). The four bands, absent from the spectra of uncoated jadeite, are related to the composition of the coating. The 2873 and 2928 cm−1 bands are caused by the symmetric and asymmetric stretching vibrations of CH2 in epoxy resin, while the 2958 cm−1 infrared absorption band caused by the asymmetric stretching vibration of CH3 in epoxy resin is relatively weak (Fritsch et al., 1992; Qi et al., 2005). The results indicate the existence of an epoxy resin. The IR spectra of the coated and uncoated jadeite can be seen in figure 9.
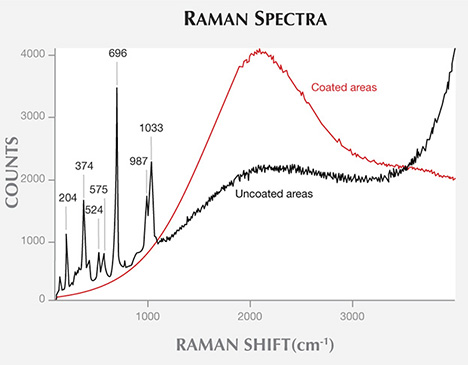
In figure 10, Raman spectroscopy of the samples’ coated and uncoated areas reveals fingerprint spectral bands in areas where the coating has chipped away. The most prominent Raman feature of uncoated jadeite is the very strong sharp band near 700 cm−1, attributed to symmetric Si-O-Si stretching (Shurvell et al., 2001; Prencipe, 2012). Other characteristic features are a doublet, consisting of a strong band near 1040 cm−1 plus a band of lower intensity near 990 cm−1, and a strong band near 375 cm−1 related to Al-O stretching or O-Si-O deformation. This band usually has a shoulder on the high-wavenumber side. The strong peak at 1033 cm−1 is due to asymmetric stretching, while the lower-intensity peak at 987 cm−1 is attributed to Si-Onb,br (representing non-bridged and bridged) stretching (Shurvell et al., 2001; Prencipe, 2012). In addition, absorption peaks at 204, 524, and 575 cm−1 were observed in the Raman spectra. These bands were absent in the areas covered by the coating. The coated area showed only a strong broad band near 2000 cm−1, which may be caused by the coating’s strong fluorescence background in the Raman spectra.
CONCLUSION
Classic gemological methods are useful in identifying jadeite that has undergone a coating process. Samples treated in this fashion are likely to have a warm feel, a relatively low hardness, and a low RI. But the definitive tests are microscopy, DiamondView imaging, and infrared spectroscopy. Observed under magnification, coated jadeite often displays traces of scratching, and the attractive green color is concentrated only in the surface layer. When examined with the DiamondView, the coating fluoresces slightly blue with alternating brighter spots. The presence of a very intense group of peaks at 2856, 2873, 2928, and 2958 cm−1 in the mid-infrared region may be characteristic of a coating that contains epoxy resin.
The coated and uncoated areas were clearly distinguished by laser scanning confocal microscopy, which can also measure the thickness of the coating. This technique has also been used to identify and measure the coating thickness of diamond, tanzanite, and cubic zirconia (Shen et al., 2007; McClure and Shen, 2008; Shigley et al., 2012). In addition, the visible-range and Raman spectra showed distinct bands for the coating and the material below.
.jpg)


