Gemological Characteristics of Saltwater Cultured Pearls Produced After Xenotransplantation
ABSTRACT
Experimental saltwater cultured pearls produced after xenotransplantation between P. margaritifera and P. maxima were studied using UV-Vis-NIR and PL spectroscopy as well as radiography. The results further demonstrate that the graft (saibo) largely determines the coloration and nacre thickness of the cultured pearl.
The value of beaded saltwater cultured pearls (SWCPs) depends on five main factors: shape, size (diameter and nacre thickness), color (bodycolor and overtone), luster, and surface condition (Taylor and Strack, 2008; Tayale et al., 2012). Statistics have shown that only 5% of all SWCPs are top quality, yet these account for about 95% of a pearl farm’s income (Haws, 2002). To increase the percentage of top-quality SWCPs, several authors have experimented with variables such as environmental factors and the choice of donor and acceptor mollusks (see examples in Lucas, 2008; Southgate, 2008; and Mamangkey 2009).
Most saltwater pearls are cultivated after transplantation of a piece of mantle tissue. This graft, also known by the Japanese term saibo, is cut from a bivalve mollusk donor. A bead, usually from the inner shell of a freshwater mollusk belonging to the Unionidae family, is simultaneously implanted into the gonad of a bivalve mollusk acceptor or host. When the donor and the acceptor bivalves belong to the same species, as is generally the case, the process is known as allotransplantation. Allotransplanted mollusks of Pinctada maxima typically produce white to light gray, silver, cream, and yellow to golden SWCPs. Allotransplanted mollusks of Pinctada margaritifera commonly yield dark gray to black as well as light gray to white SWCPs. Various other natural-color SWCPs can be also found in both bivalves (see Karampelas et al., 2011 and 2012, and references therein).
McGinty et al. (2010 and 2011) presented the results of their genetic studies involving successful xenotransplantation between two different species (P. margaritifera and P. maxima) and the influence on the aforementioned SWCP quality factors. This study investigated experimental SWCPs, using methods different from those presented by McGinty et al., to further confirm the effect of the saibo from the donor mollusk.
MATERIALS AND METHODS
This study was carried out on 10 successfully cultivated experimental SWCPs (selected from McGinty et al., 2010) with various colors and sizes (see figure 1 and table 1). Seven samples (nos. 1–7) were cultivated in P. maxima after transplantation of a P. margaritifera tissue graft, while the other three (nos. 8–10) were cultivated in P. margaritifera after transplantation of a P. maxima graft. All samples were cultivated for 14 months on a farm belonging to Cendanda Indopearls on the Indonesian island of Bali; more on the exact conditions of cultivation can be found in McGinty et al. (2010). None of them had been subjected to any treatment. All but sample 9 were round or near-round, with good to very good surface condition and mostly good luster; see Gübelin Gem Lab (2012) for more information about the grading system used. SWCPs cultivated in P. maxima mollusks with P. margaritifera grafts had more grayish color than those cultivated in P. margaritifera mollusks with P. maxima grafts (again, see table 1).
Figure 1. Ten xenografted saltwater cultured pearls were chosen for this study. Seven samples (nos. 1–7) were cultivated in P. maxima with transplanted P. margaritifera tissue graft, while the other three (nos. 8–10) were cultivated in P. margaritifera with transplanted P. maxima graft. Composite photo by S. Karampelas.
RESULTS AND DISCUSSION
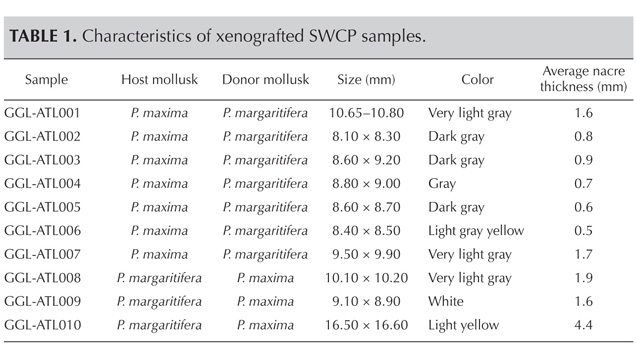
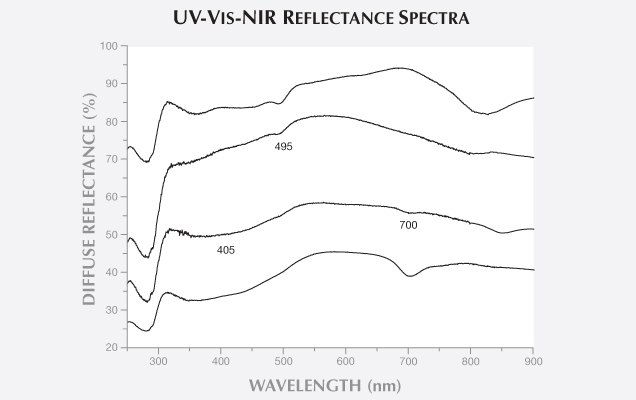
Figures 2–4 show the diffuse reflectance UV-Vis-NIR spectra for six xenotransplanted samples. The spectra present an absorption (a decrease in diffuse reflectance) at around 280 nm. Figure 2 shows two natural-color samples cultivated after xenotransplantation into P. maxima mollusks with P. margaritifera grafts, GGL-ATL002 (dark gray) and GGL-ATL004 (gray), as well as one black natural-color SWCP from P. margaritifera after allotransplantation (bottom spectrum). All three spectra contain six main absorption bands: from 330 to 460 nm, with maxima at 330–385 nm and 385–460 nm, and at 405, 495, 700, and 745 nm (plus a continuous band extending through the visible range with a maximum in the near infrared at around 820 nm). Also observed are three less-intense bands at around 530, 585, and 625 nm, which are common in allotransplanted P. margaritifera SWCPs (Elen 2002; Karampelas et al., 2011). Differences in the spectra patterns are due to the different relative intensities of these bands. The 700 nm band is currently known only from allotransplanted P. margaritifera SWCPs (Elen, 2002). Moreover, the 405 nm band has not been observed in natural-color allotransplanted P. maxima SWCPs (Karampelas, 2012). These results are in accordance with those found experimentally by McGinty et al. (2010 and 2011), as well as other authors (e.g., Wada and Komaru, 1996). In other words, the saibo—in this case, P. margaritifera tissue—is mainly responsible for the coloration of the SWCPs. None of these bands is linked to a specific pigment, except for the one at approximately 405 nm, which is attributed to a kind of porphyrin (Iwahashi and Akamatsu, 1994).

CONCLUSION
Xenotransplantation between P. margaritifera and P. maxima can yield gem-quality SWCPs, as documented by McGinty et al. (2010 and 2011). This study using UV-Vis-NIR spectroscopy as well as radiography confirmed the histological and genetic findings by various researchers (e.g., Arnaud-Haond et al., 2007; McGinty et al., 2010) that the saibo from the donor mollusk is mainly responsible for the color as well as the nacre thickness. Using spectroscopic means, gemological laboratories can identify (with the exception of some light-colored SWCPs) the mollusk species of the donor (e.g., the 700 nm absorption band characteristic of saibo from P. margaritifera) but not the host. The host mollusk probably plays some role in the nacre deposition. For instance, xenotransplanted SWCPs with a P. margaritifera host and saibo from P. maxima have slightly thicker nacre than the allotransplanted SWCPs from P. maxima (McGinty et al., 2010). Additional research is needed to shed light on this.Moreover, several studies have shown that selecting the best-secreting saibo for transplantation into a healthy host mollusk is the key to SWCP quality (e.g., Acosta-Salmón et al., 2004; Southgate, 2008). Further research is also needed on all five quality factors in xenografted SWCPs, including comparison with allografted SWCPs from the same mollusk species under identical conditions, after careful selection of donor and host mollusks. These investigations would clearly show if quality can be improved through xenografting. Another meaningful experiment, suggested by various authors, would be to see if xenografting between other Pinctada species (e.g., P. fucata) or even related species (e.g., Pteria sp.) can yield high-quality SWCPs.



