Overview
Tourmaline’s colors have many different causes. It’s generally agreed that traces of iron, and possibly titanium, induce green and blue colors. Manganese produces reds and pinks, and possibly yellows. Some pink and yellow tourmalines might owe their hues to color centers caused by radiation, which can be natural or laboratory-induced.
Birthstones & Anniversaries
Tourmaline is a birthstone for October, along with opal. Tourmaline is also the gem of the eighth anniversary.
1554
Francisco Spinoza’s expedition discovers “Brazilian emerald”: the first recorded green tourmaline crystal.
Pyroelectric
Tourmaline becomes electrically charged when heated. Also when squeezed: it’s piezoelectric too.
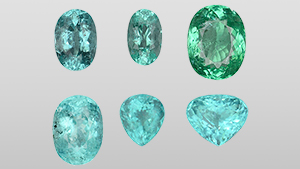
Paraiba
Brazilian source famous for vivid blue to green tourmalines colored by copper.
Facts
-
Mineral:
Tourmaline
-
Chemistry:
-
Elbaite Na(Li1.5,Al1.5)Al6Si6O18(BO3)3(OH)4
-
Dravite NaMg3Al6Si6O18(BO3)3(OH)4
-
Liddicoatite Ca(Li2Al)Al6Si6O18(BO3)3(OH)3F
-
Chromedravite NaMg3Cr6Si6O18(BO3)3(OH)4
-
Color:
All colors
-
Refractive index:
1.624 to 1.644
-
Birefringence:
0.018 to 0.040
-
Specific gravity:
3.06 (+0.20, -0.06)
-
Mohs Hardness:
7 to 7.5
Treatments
There are a number of processes used to alter the color, apparent clarity, or improve the durability of gems.
Learn MoreSynthetics
Some gemstones have synthetic counterparts that have essentially the same chemical, physical, and optical properties, but are grown by man in a laboratory.
Learn MoreImitations
Any gem can be imitated—sometimes by manmade materials or by natural materials chosen by man to impersonate a particular gem.
Learn More
Why We Love This Gemstone
Trigonal System
Tourmaline crystallizes in the trigonal system; no other common mineral has three-sided prisms.
Watermelon Variety
Watermelon tourmaline is green on the outside and a delicious pink on the inside.
Liddicoatite
Liddicoatite tourmaline was named for beloved former GIA President Richard T. Liddicoat.
Quality Factors
Tourmaline’s varieties have a wide range of quality factors considerations.
Color

Tourmaline's rainbow colors have a wide range of color intensity and tone.
Clarity

Pink to red tourmaline often has more visible inclusions than green to blue varieties.
Cut

Tourmaline crystals are often long, leading cutters to cut slender finished stones.
Carat Weight

Tourmalines come in all shapes and sizes. The value change for size varies with the variety.
Tourmaline Quality Factors: The Comprehensive Guide
Research
Explore sources, gemological research, and the role of gems in history.


Expedition to the Cruzeiro Tourmaline Mine in Minas Gerais, Brazil
Andrew Lucas, Duncan Pay, Tao Hsu, Shane McClure, and Pedro Padua , Mar 27, 2015 Read Article
Solution-Generated Pink Color Surrounding Growth Tubes and Cracks in Blue to Blue-Green Copper-Bearing Tourmalines from Mozambique
John I. Koivula, Kevin Nagle, Andy Hsi-Tien Shen, and Philip Owens , Apr 6, 2009 Read ArticleRecommended Reading

Paraiba Tourmaline: “Electric Blue Brilliance Burnt Into Our Minds”
Masashi Furyuya

Collector’s Guide to the Tourmaline Group
Robert J. Lauf

Tourmaline: A Gemstone Spectrum
Brian C. Cook















.jpg)


