HPHT-Processed Natural Type Ia Diamond

There are two types of high-pressure, high-temperature processes related to diamond: HPHT treatment and HPHT diamond growth. HPHT laboratory-grown diamonds were developed in the mid-1950s, with the first gem-quality production in the early 1970s and extensive production beginning in the 1990s.
HPHT treatment to change the color of natural diamonds was discovered as a byproduct of annealing natural diamond anvils. The General Electric Company (GE) and Lazare Kaplan International marketed the first commercial HPHT-treated color diamonds in March 1999 (K. Schmetzer, “Clues to the process used by General Electric to enhance the GE POL diamonds,” Winter 1999 G&G, pp. 186–190). The same machines, such as a hydraulic press that produces extremely high pressure and temperature environments, are also used to treat both mined and laboratory-grown diamonds. HPHT treatment can change the color of diamonds or make them colorless.

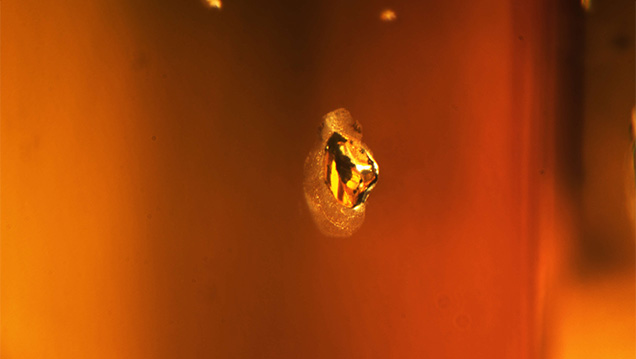
A natural 5.20 ct type Ia SI2 Fancy Dark yellowish brown diamond with natural anhedral crystal inclusions was submitted to the New York laboratory for grading services and returned. After being treated, it was resubmitted as a 5.17 ct type Ia SI2 Fancy Deep yellow-orange diamond (figure 1), with the disclosure that it had been HPHT processed. After analysis, the diamond was confirmed to have been treated using an HPHT process that changed the color from a dark yellowish brown to a deep yellow-orange (figure 2). After the HPHT process, each of the natural anhedral crystal inclusions developed a graphitized halo (figure 3). In the natural diamond, the visible/near-infrared (Vis-NIR) absorption spectrum showed a slight 550 nm band—a feature that may impart a pinkish color component depending on its absorbance and other features present within the diamond. After HPHT treatment, there is instead an increase in absorption from ~500 nm toward the lower wavelengths; this absorption rise is due to the creation of single, isolated nitrogen (figure 4).


As expected (I.M. Reinitz et al., “Identification of HPHT-treated yellow to green diamonds,” Summer 2000 G&G, pp. 128–137), FTIR data from after the HPHT treatment showed that a peak associated with isolated nitrogen at 1344 cm–1 was introduced, the ~1363 cm–1 platelet peak was diminished, and the natural amber center at ~4165 cm–1 was destroyed (figure 5). Since the spectroscopic data changed after treatment, crystal inclusions plotted on the original grading diagram for this diamond confirmed it was the same stone. The 550 nm band in the Vis-NIR absorption spectrum related to a pink or brown diamond is stable at lower temperatures of HPHT treatment. Since the 550 nm band was not detected after the process, we believe this diamond was treated at the higher end of the HPHT regime or for a prolonged duration that required repolishing, which caused it to lose three points of carat weight.
While there are many different treatments that can be performed on diamonds, HPHT treatments are permanent and require a stable and controlled environment. In this stone, the treatment resulted in some of the nitrogen aggregates being split apart into single, isolated nitrogen that introduced new color centers and altered the color of the treated stone. The treatment also graphitized inclusions; however, this change was not sufficient to lower the clarity grade. As technology advances, HPHT treatments are becoming more common for both natural and laboratory-grown diamonds.
.jpg)


