Observations on HPHT-Grown Synthetic Diamonds: A Review

ABSTRACT
This article presents statistical data and distinctive features for several thousand HPHT-grown synthetic diamonds examined by GIA from 2007 through 2016. This study, the first comprehensive summary published on such a large number and wide variety of samples, describes the reliable means of identifying them, with a focus on material currently marketed for jewelry use. The color of HPHT synthetic diamonds analyzed by GIA has shifted noticeably during this time—in the early years, orange-yellow, yellow, and yellow-orange samples comprised the overwhelming majority, while colorless and blue samples are much more prevalent today. HPHT synthetics are making inroads into the large diamond market, with cut stones larger than 10 carats, as well as the colorless melee market, where small HPHT synthetics are being mass-produced in China. HPHT synthetics can be identified by their distinctive fluorescence patterns using the DiamondView luminescence imaging instrument, the lack of “strain” (anomalous birefringence) when viewed through crossed polarizers, and to a lesser extent by the detection of various features in photoluminescence (PL) spectroscopy. This material may also display magnetism and a short-wave fluorescence and phosphorescence reaction that are inconsistent with similarly colored natural diamonds.
INTRODUCTION
Synthetic gem diamonds grown by the high-pressure, high-temperature (HPHT) process have been commercially available since the mid-1990s. This article presents statistical information and distinctive identification features based on a review of data gathered by GIA, principally at the New York and Carlsbad laboratories, for several thousand HPHT-grown synthetic diamonds. This study includes all HPHT synthetic diamonds submitted to GIA between 2007 (the year GIA started issuing Synthetic Diamond Grading Reports) and 2016. No summary has been published on such a large number of HPHT synthetic samples. We describe here the diagnostic means of identification, with an emphasis on the goods currently being sold for jewelry use. Box A details some of the most important identification criteria that may be used by gemologists.
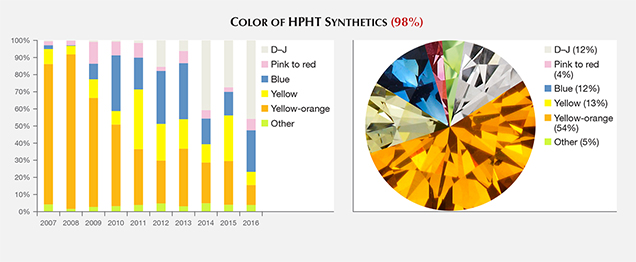
Of this sample set, 12% were colorless to near-colorless (D–J), 12% blue, 13% yellow, 4% pink to red, and 54% yellowish orange to orangy yellow (e.g., figure 1). The remaining 5% showed other colors, including green-yellow and brown-orange. This set represents both as-grown and treated colors. While some samples were purchased by GIA on the market or from manufacturers, or were loaned or donated to us by manufacturers for study purposes, most were submitted to GIA in polished form for identification or grading reports. To the best of our knowledge, this sample set is representative of the gem-quality HPHT synthetic material available in the trade.
The evolution of HPHT synthetics has been detailed in previous G&G articles (Shigley et al., 2002, 2004; D’Haenens-Johannsson et al., 2014, 2015) and Lab Notes entries (e.g., Ardon and Batin, 2017; Johnson et al., 2017) that include diamonds within this dataset. Whereas earlier studies examined small batches of material from specific manufacturers (which were likely grown using a similar recipe or HPHT pressure equipment), the goal of the present study is to investigate trends in the distinctive features seen among HPHT synthetic diamonds from multiple sources over the past ten years.
During the decade covered in this review, the colors of HPHT synthetics have shifted from predominantly yellow and orange to colorless and blue. The early years (2007–2008) likely represent the colors manufactured during the prior decade. The high number of submissions in those early years represents material that had already been available in the trade—for example, synthetics in the yellow, green, pink, and blue color ranges discussed by Shigley et al. (2004). There has also been a dramatic expansion in the size ranges. This includes polished gems greater than 10 carats (although production is still limited), as well as a significant increase in the output of HPHT synthetic melee, whose volume and small stone size present particular identification problems for the jewelry trade.
HPHT SYNTHETIC DIAMOND GROWTH
High-pressure, high-temperature diamond growth was first announced by the General Electric Company (GE) in 1955, and today most HPHT synthesis has its origins in the temperature gradient method first developed in 1959 (Bovenkerk et al., 1959). Yet it was only during the 1990s that these products attained sufficient size and gem quality to present significant concerns to the jewelry industry (Shigley et al., 1997). High-pressure reactor chamber design and control of diamond growth parameters have advanced considerably in recent years. The consistent production of larger as well as colorless HPHT synthetics is now possible, and several HPHT synthetic crystals can be grown simultaneously within the press (D’Haenens-Johansson et al., 2015). In general, HPHT growth proceeds at temperatures and pressures designed to approximate the conditions of natural diamond growth. Within the earth, diamonds generally form at pressures of 5.5–8.0 GPa (55,000–80,000 atmospheres) and temperatures of 1000–1400°C, corresponding to depths of 140–240 km (Shirey and Shigley, 2013).
In HPHT growth, a carbon source such as graphite or diamond powder is placed in the reactor chamber along with other ingredients to facilitate diamond growth atop a diamond seed. A molten metal catalyst (usually containing a mixture of Fe, Ni, Co, or other elements) allows growth to proceed at a lower temperature. This also reduces the technological complexity and some of the expense required to grow diamond under HPHT conditions. HPHT growth occurs at pressures of 5–6 GPa (roughly equivalent to the pressure exerted by a commercial jet airplane if balanced on the tip of a person’s finger) and at temperatures of 1300–1600°C. As with CVD diamond growth, HPHT growth proceeds by creating a temperature gradient in which the carbon source is at a slightly higher temperature than the diamond growth seed. This causes the carbon atoms to diffuse through the molten flux toward the slightly colder section of the chamber to form a synthetic diamond crystal on the seed. Most early gem-quality HPHT synthetics were fancy color, since color-causing impurities, such as nitrogen (yellow) or boron (blue), were often prevalent within the growth system. Recent progress in growth technology has allowed for better control of impurity contents, resulting in the creation of colorless crystals (e.g., figure 2).

ANALYSIS OF EXISTING DATA
We will summarize quality factors, basic gemological properties, ultraviolet fluorescence reactions, and key spectral features based on information gathered when these several thousand HPHT-grown samples were examined by GIA between 2007 and 2016. In some instances, GIA did not collect all data types on a particular HPHT synthetic sample for various reasons: time constraints, the lack of certain instrument capabilities at the time of examination, or the need to limit the analysis to the grading service requirements requested by the submitting client. In the following discussion, therefore, we will indicate the percentage of total submissions for which that data was collected. Nonetheless, the information reported here represents the most substantial database of observations published to date on gem-quality HPHT synthetic diamonds.
We noted no significant differences in characteristics between the HPHT synthetic samples sourced directly from the manufacturers and those submitted by GIA laboratory clients. The information presented here is strictly limited to HPHT synthetics examined by GIA, which might not represent all of the goods available within the gem trade and certainly not those crystals grown for technological or industrial applications.
ANALYSIS OF QUALITY GRADING FACTORS
Color. Since 2007, there has been a pronounced shift in the color of the HPHT synthetics submitted for grading. In the early years of GIA’s Synthetic Diamond Grading Report, the overwhelming majority were orangy yellow to yellowish orange (in this article, this color range is shortened to “yellow-orange”; e.g., figure 3). The proportion of HPHT synthetics with pink coloration has remained generally constant at around 3–8%. During the early years, blue samples were not submitted in large numbers. Since then, the blue and yellow hues have shown some fluctuations in annual percentage but exhibited no distinct trends. Colorless to near-colorless synthetics have shown a dramatic increase, now reaching 43% of the HPHT synthetics submitted to GIA in 2016. While the proportion of yellow-orange HPHT synthetics that have been submitted (and likely manufactured) has decreased in recent years, they still comprise a clear majority of the HPHT synthetics submitted to GIA from 2007 through 2016 (figure 3, right).
Among the colorless to light yellow (D–Z) HPHT synthetics, the vast majority (72%) were in the colorless (D–F) range, while 26% were in the near-colorless (G–J) range, and the remainder had lower color grades. In contrast, most D–Z CVD synthetics examined by GIA were in the near-colorless range: 21% D–F, 67% G–J, and 10% K–N (Eaton-Magaña and Shigley, 2016).
Most colors of HPHT synthetics are believed to be as-grown (i.e., due to impurities) rather than the product of post-growth treatment processing. In contrast, CVD synthetics are often subjected to post-growth treatment. Most near-colorless CVD synthetics are subsequently HPHT treated (to remove any brown coloration), while the pink samples (the other major CVD product) undergo irradiation and annealing (Eaton-Magaña and Shigley, 2016).
The colors of “yellow-orange” and yellow HPHT synthetics are due to the presence of isolated nitrogen incorporated during the growth process (Shigley et al., 2002); however, the additional use of “nitrogen-getters” such as aluminum in the growth system can reduce the incorporation of nitrogen (Sumiya and Satoh, 1996). Almost all “orange” HPHT synthetics included a yellow component, while some contained a brown or pink modifier; very few were pure orange.
“Pink” HPHT synthetics (including purple-pink, red, and brown-red) owe their color to post-growth treatment by irradiation and low-temperature annealing. This procedure creates nitrogen-vacancy (NV) optical centers, which are also the source of color in treated pink diamonds, treated CVD synthetics, and the rare natural and untreated Golconda pinks.
Blue HPHT synthetics result from the presence of boron within the growth chamber. Colorless HPHT synthetics are reportedly sold as-grown without post-growth color modification (D’Haenens-Johansson et al., 2014), and they often contain small amounts of boron, which does not alter their color (but does impart luminescence).
Other less common colors include yellow-green to green samples. Green coloration has a number of origins in HPHT synthetics, including the presence of yellow zones (due to nitrogen) and blue zones (due to boron) that visually combine to give a greenish appearance (Shigley et al., 2004); laboratory irradiation after growth (Shigley et al., 2004); and high quantities of nickel impurities (Johnson et al., 2017).
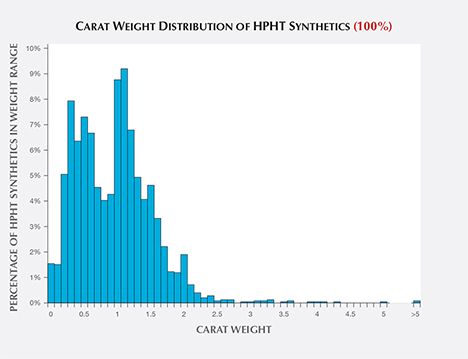
Carat Weight. Faceted HPHT synthetics in the 1.00–1.50 ct range were the most common, representing 34% of those examined by GIA (figure 4). While HPHT synthetics larger than four carats have been newsworthy during the last few years, relatively few have been submitted, presumably due to very limited production. Additionally, the carat range distribution has not varied considerably from 2007 to 2016. Production of colorless melee has increased greatly in recent years (W. Wang, pers. comm., 2016), but so far no fancy-color synthetics of any size have been submitted to GIA in large numbers. With the announcement in late 2016 of a colorless melee sorting service, GIA has seen more of these very small synthetic diamonds; for more on HPHT synthetic melee, see box B.
Since 2015, many of the previous records for carat weights of polished synthetic diamonds have been shattered (Deljanin et al., 2015; Wang and Poon, 2016). The Russian company New Diamond Technology has provided several colorless to near-colorless diamonds to GIA greater than five carats, along with several blue samples. This advancement has generated excitement as well as concern in the jewelry trade.
Cut. The majority of the faceted HPHT synthetics analyzed were round brilliants (51%). Other common styles included square (19%) and rectangle (18%), with and without cut corners (figure 5, top). Manufacturers appear to have chosen the round brilliant cut because of the popularity of this style, even though a “fancy” shape would have retained slightly more weight from the original crystal.
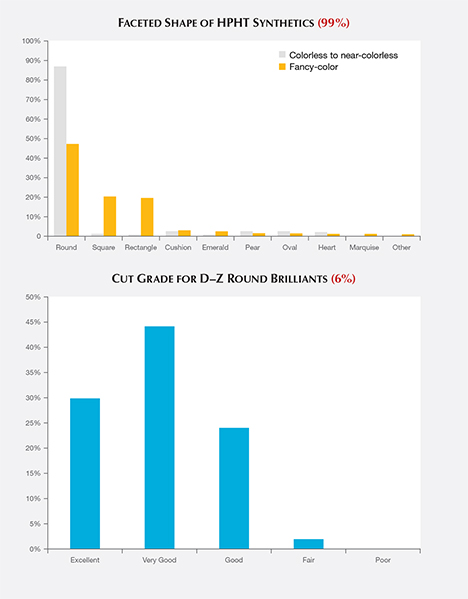
Since GIA only assigns cut grades for D–Z color round brilliants, only a small percentage of the HPHT synthetics were eligible for such an assessment. Among these, 44% received a Very Good cut grade, with 30% Excellent and 24% Good (figure 5, bottom).
Clarity. The HPHT synthetics span nearly the entire clarity scale, but most received grades in the VS1/VS2 range (figure 6). This is slightly lower than the clarity range observed for CVD synthetics, most of which were in the VVS2/VS1 range, and slightly higher than the VS2/SI1 range observed for colorless to near-colorless natural diamonds (Eaton-Magaña and Shigley, 2016). As GIA generally examines only the material that has been released by HPHT manufacturers as worthy of cutting, the samples likely represent what is available within the trade but might not account for the entire manufacturing output.
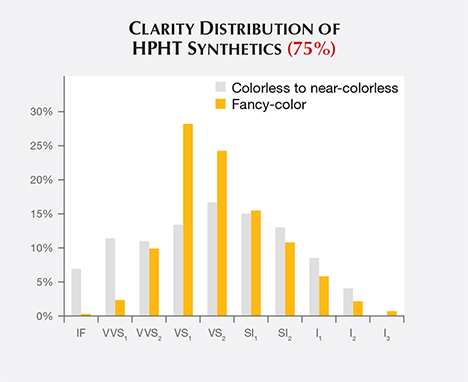
The clarity distribution is shifted toward higher grades among colorless synthetic diamonds than among fancy-color samples. This may be due to the different chemistry in the HPHT presses that create colorless material, or more likely the fact that most colorless goods date from recent years (figure 3, left) and represent improvements in the control of diamond crystallization.
Clarity grading of diamonds often involves the determination of the “grade-setting” clarity feature. This is the visual characteristic seen at 10× magnification that determines the clarity grade. For example, a diamond might have a large feather and a few sparse pinpoints. While both would be listed as inclusions, the feather would be considered the “grade setter.” A number of different grade-setting clarity characteristics were found in these HPHT synthetics (figure 7).
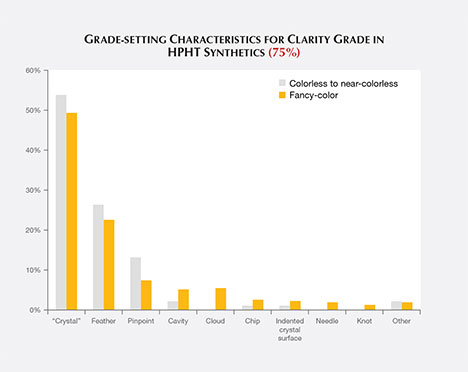
The most common grade-setting inclusions for HPHT synthetic diamonds were identified by laboratory graders as “crystals.”1 The other most common features were feathers, pinpoints, and clouds. These inclusions were mentioned in previous articles (Shigley et al., 2002, 2004; D’Haenens-Johansson et al., 2014, 2015). Many of these features also contained radial fractures surrounding larger inclusions; fractures that reached the surface are plotted as feathers.

ANALYSIS OF OTHER GEMOLOGICAL PROPERTIES
Long-Wave and Short-Wave Fluorescence. While it should not be considered conclusive evidence, the observation of ultraviolet fluorescence with a standard long-wave/short-wave UV unit has been an important and practical means of distinguishing natural diamonds from HPHT-grown synthetics. The determination is based on differences in fluorescence colors, intensities, and patterns (and in some cases the occurrence of persistent phosphorescence). If a fluorescence reaction is observed in an HPHT synthetic, the reaction to short-wave UV radiation is often stronger than to long-wave UV radiation (or the reaction to long-wave is altogether absent). For HPHT synthetics in our dataset that had both long- and short-wave fluorescence reactions, 11% showed a stronger long-wave UV reaction, 32% had equal-intensity fluorescence reactions, and 58% had a stronger short-wave reaction.
The bar graphs in figure 9 (top) illustrate the intensity of fluorescence reactions to long-wave and short-wave ultraviolet radiation from a standard gemological UV lamp. Most HPHT synthetics displayed no fluorescence reaction to long-wave UV (across all colors, 74%), while a significantly lower percentage (38%) had no reaction to short-wave UV. While the observed fluorescence intensity was generally greater in response to short-wave rather than long-wave UV, the distribution of fluorescence color was quite similar (figure 9, bottom).
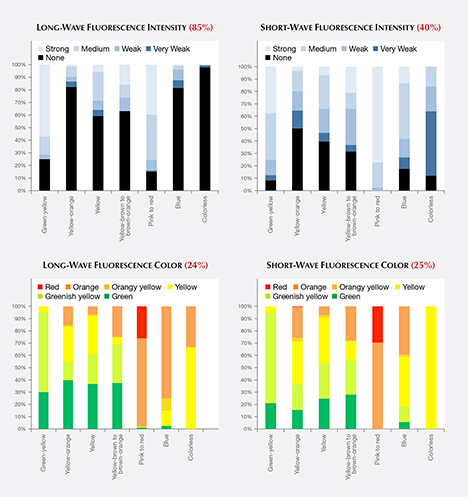
Fancy-Color Material. Among pink to red HPHT synthetics, where the color origin and the fluorescence color are due to the presence of the NV defect centers acquired during post-growth treatment, the majority showed moderate to strong fluorescence, typically with orange or red colors. Only 15% had no reaction to long-wave UV, and all pink HPHT synthetics exhibited an observable reaction to short-wave UV. In comparison, 16% of the natural pink diamonds had no reaction to long-wave UV, and 31% had no reaction to short-wave UV (King et al., 2002). Among those natural diamonds with observed fluorescence, the vast majority gave a blue reaction, while the remainder showed yellow or rarely orange fluorescence. Only a very small percentage (<1%) of natural pink diamonds derive their color from NV centers, and it is this small percentage that showed orange rather than blue fluorescence.
Among yellow to yellow-orange material, the percentage of HPHT synthetics showing a fluorescence reaction to long-wave UV was lower than for short-wave. The observed fluorescence colors were typically green to orange. This color range was distinctly different from those observed in natural diamonds, in which the most common color of long-wave fluorescence was blue (92%; King et al., 2005).
Colorless Material. Only 2% of colorless HPHT synthetics showed a fluorescence reaction to long-wave UV. This is a far lower percentage than observed among natural diamonds. Moses et al. (1997) reported that 35% of natural diamonds in the same color range had detectable long-wave fluorescence, and that 99% of the fluorescence reactions were blue due to the N3 optical defect combined with “band A” luminescence (Zaitsev, 2003). By contrast, the small percentage of HPHT synthetic diamonds with detectable long-wave fluorescence emitted orange and yellow colors.
Meanwhile, a much higher percentage (88%) of colorless HPHT synthetics showed a fluorescence reaction to short-wave UV (figure 9). This reaction is consistent with fancy-color HPHT synthetics (Shigley et al., 2004) and prior observations of colorless HPHT synthetics (D’Haenens-Johansson et al., 2014), and opposite the expected reaction for natural diamonds (Shigley et al., 1993).
Short-Wave UV Phosphorescence. HPHT synthetics in the blue and colorless color ranges often show observable light blue phosphorescence (e.g., D’Haenens-Johansson et al., 2014, 2015) due to boron impurities (Watanabe et al., 1997). Treated and natural blue type IIb diamonds often showed blue phosphorescence as well, though typically weaker (natural type IIb diamonds can also show red phosphorescence; Eaton-Magaña and Lu, 2011). Among the other HPHT synthetic diamond colors, the majority did not exhibit observable phosphorescence. For example, only one of the yellow-orange samples showed phosphorescence (weak orange).
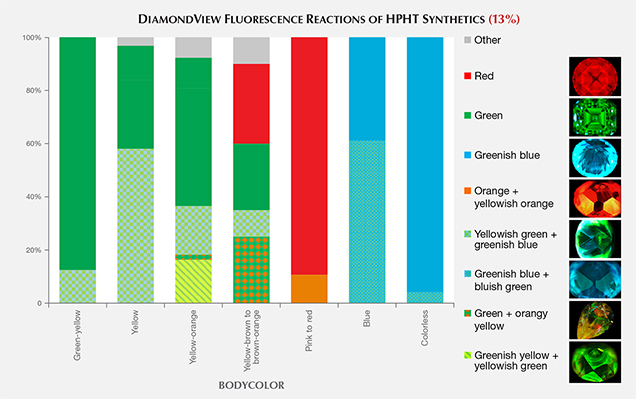
DiamondView Imaging. For the past two decades, the DiamondView fluorescence imaging instrument has been an important diamond identification tool for major gemological laboratories. The design of the sample holder allows diamonds to be positioned and examined in various orientations, and the fluorescence reactions can be observed in real time. The very short wavelength (~225 nm) and high intensity of the UV excitation source creates fluorescence just beneath facet surfaces, producing a distinct reaction. Although most HPHT synthetics show no reaction to a standard long-wave UV light source and many are inert to short-wave UV, all diamonds—including all HPHT synthetics—show some observable reaction to the high-intensity, high-energy UV source.
Differences in UV fluorescence colors and patterns provide the basis for DiamondView analysis (Welbourn et al., 1996). Because of their unique growth environment, HPHT synthetics exhibit distinctive fluorescence reactions under the DiamondView.
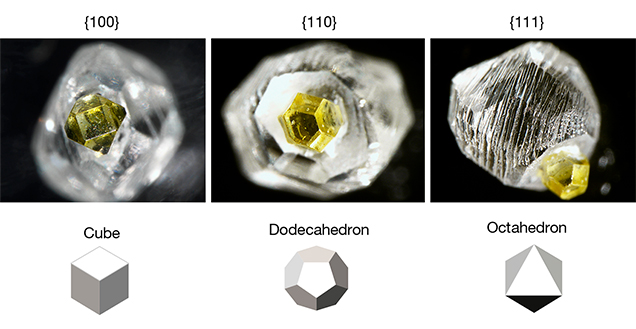
Growth Features. The growth features of HPHT synthetics are created largely by the prevailing growth chemistry and process conditions that have existed for several decades. In order to lower the temperature and pressure necessary for HPHT growth, as well as the associated expense, manufacturers use a metal catalyst (see “HPHT Synthetic Diamond Growth” above). At these lower temperatures, octahedral growth (as seen in natural diamond crystals) is not observed (Welbourn et al., 1996). Instead, cuboctahedral growth predominates (at even lower temperatures, cube growth would occur). The vast majority of distinctive growth features seen in HPHT synthetics are due to this cuboctahedral arrangement of internal growth sectors, as well as accompanying variations that can occur with different metal catalysts (Welbourn et al., 1996).
The two as-grown HPHT synthetic crystals in figure 10 demonstrate the characteristic external crystal morphology. Their corresponding DiamondView fluorescence images show the different fluorescence reactions along these growth faces. The impurities and defects created in HPHT synthetics can vary greatly depending on the growth face, and these variations are revealed by the different fluorescence colors. Once an HPHT synthetic is polished, the as-grown morphology can no longer be easily observed unless there is noticeable color zoning. However, the differences in defect chemistry within these growth faces are preserved even in colorless material, with the fluorescence reaction and pattern revealed quite clearly by DiamondView imaging.
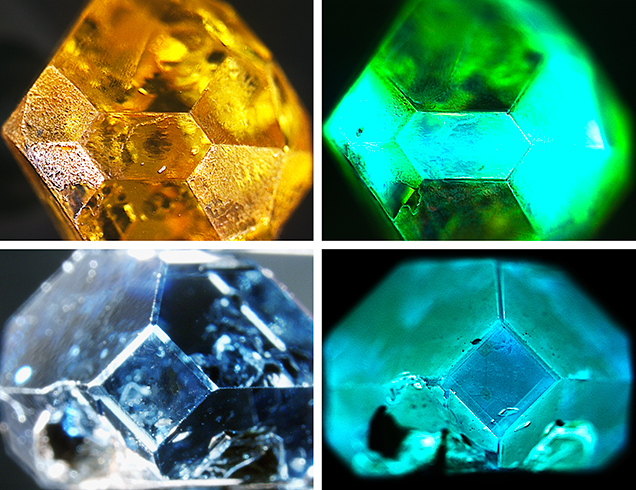
Fluorescence Color. With the DiamondView, a wide variety of fluorescence colors can be observed in HPHT synthetics. HPHT synthetics often exhibit more than one fluorescence color (e.g., green and greenish blue in figure 10, top); examples of the fluorescence colors are shown in figure 11.
Fancy-color diamonds generally show a contrast in fluorescence color between the various growth planes. Often the planes reveal two distinct fluorescence colors, or a combination of regions with and without fluorescence. The green fluorescence is ascribed to H3 defects, while the orange to red fluorescence originates from NV centers. The origins of the other fluorescence colors are less understood by scientists. A comparison of these fluorescence colors against other known spectral features did not indicate any compelling correlations.
With colorless HPHT synthetics, the contrast in fluorescence color between growth sectors is much more subtle (D’Haenens-Johansson et al., 2014) and can be difficult for a gemologist to detect even while rotating the sample in the DiamondView. For colorless diamonds in particular, the gemologist must be especially vigilant when looking for growth features in the DiamondView. This reduction in color contrast is due to fewer defects and color-causing impurities in colorless as compared to fancy-color HPHT synthetics. Therefore, the concentration differences of defects between the growth sectors that create the fluorescence will not be as apparent.
DiamondView Reaction Compared with Short-Wave and Long-Wave UV Fluorescence. Figure 12 shows the fluorescence reaction observed for two fancy-color HPHT synthetics. The series in figure 12 (top) clearly indicates the hourglass growth zoning indicative of HPHT synthetic growth when illuminated by the deep ultraviolet (~225 nm) illumination of the DiamondView. Using short-wave UV, the hourglass pattern is still visible, but not as distinct. While the HPHT synthetic exhibits some reaction to long-wave UV, there is no observable pattern.
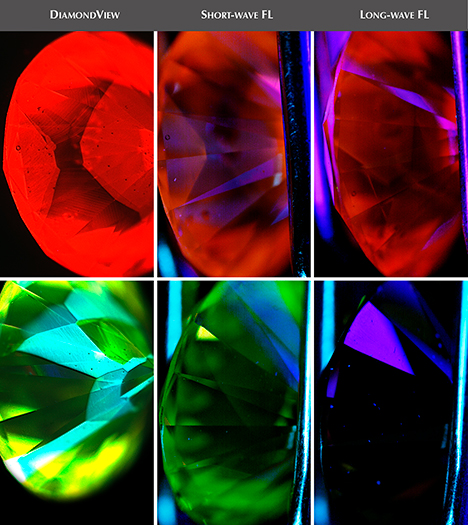
Far more common among HPHT synthetics is the sample in figure 12 (bottom), in which the DiamondView shows a reaction that is distinctive of HPHT synthetics while the short-wave and long-wave UV fluorescence pattern are not at all diagnostic of HPHT growth. The superior detail and pattern contrast of DiamondView imaging has made the instrument indispensable in gemological laboratories, as the quality of data cannot be matched using short-wave or long-wave UV lamps. Published reports on synthetic diamonds often include fluorescence reactions observed with the DiamondView, so the gemologist should understand that such reactions are less intense and less obvious using a standard long-wave/short-wave UV lamp.
SPECTROSCOPIC PROPERTIES
While HPHT samples have more diagnostic gemological properties than CVD samples (such as color zoning, lack of strain, and magnetism; see box A), the absence of these properties does not conclusively identify a diamond as natural. Spectroscopic techniques are essential to verify the growth origin of all diamonds. Among the samples identified as potential HPHT synthetics, a combination of features in Fourier-transform infrared absorption (FTIR), visible/near-infrared (Vis-NIR) absorption, and photoluminescence (PL) spectroscopy will confirm the determination. Therefore, positive detection of HPHT synthetics is best accomplished by a major gemological laboratory that maintains a database of known natural, treated, and synthetic diamonds and sees a sufficient quantity of goods to spot emerging trends.
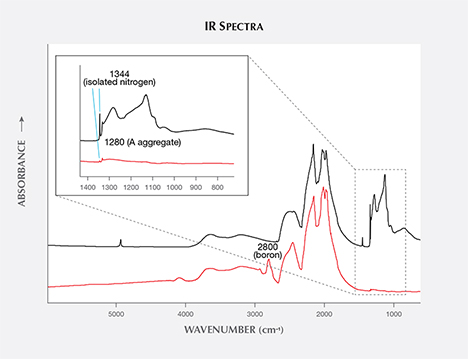
Infrared (IR) Absorption Spectroscopy. In colorless diamonds, IR absorption spectroscopy is most useful in separating type Ia, which represents the vast majority of natural diamonds, from diamonds that do not contain aggregated nitrogen (Breeding and Shigley, 2009). This is the basis of GIA’s DiamondCheck instrument. This distinction is important in colorless diamonds, as only those without detectable aggregated nitrogen are identified as potentially treated or synthetic.
Among fancy-color HPHT synthetics, either with as-grown yellow-to-green or treated pink colors, IR absorption spectra display the 1344 cm–1 peak. This feature indicates the presence of single substitutional nitrogen (i.e., type Ib diamond) and shows that the diamond is potentially quite young in geologic terms. While some natural diamonds can also be type Ib, this peak is a good indicator of potential HPHT synthetic origin, as young diamonds contain only isolated nitrogen. In natural stones, over the millions of years that diamonds reside at higher temperatures in the earth, these isolated nitrogen atoms randomly migrate within the diamond lattice (figure 13) and inevitably find another nitrogen defect to form what are called nitrogen aggregates. The A aggregate (a pair of substitutional nitrogen atoms in adjacent lattice sites) can also combine to form the B aggregate (four nitrogen atoms surrounding a vacant atom position in the lattice). While this aggregation process can be duplicated to some extent in the laboratory using high temperature instead of millions of years, there are differences. For example, the isolated nitrogen indicated by the 1344 cm–1 peak will never be completely eliminated using high-temperature treatment, and this feature will still be detected by IR spectroscopy (Dobrinets et al., 2013). Among natural diamonds, the aggregation process over long periods of geologic time removes nearly all isolated nitrogen so that the distinct infrared spectral feature cannot be detected in most natural diamonds.
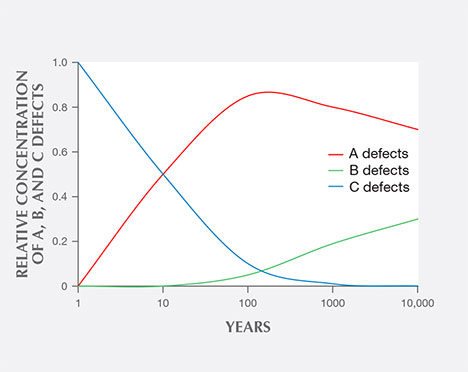
Many of our HPHT synthetic samples showed, based on their infrared spectra, a pure type Ib character (i.e., only isolated nitrogen), while others exhibited a combination of type IaA (due to heating, which created some aggregated nitrogen) and type Ib (figure 14). Whether the manufacturers are creating A aggregates by maintaining the chamber at high temperature during the growth process or by subjecting the diamonds to post-growth treatment to enhance nitrogen aggregation is not entirely clear.
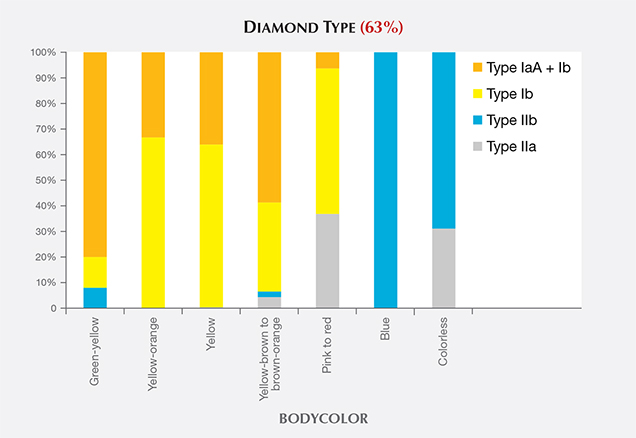
Blue HPHT synthetics (along with a majority of colorless HPHT samples) were type IIb, indicating either intentional doping or accidental contamination with boron in the growth chamber. Figure 15 shows the distribution of diamond types among HPHT synthetics based on bodycolor. Those with yellow color were predominantly type Ib or a combination of type Ib and type IaA. The blue synthetics were exclusively type IIb. Colorless HPHT synthetics were type IIa or, more commonly, type IIb (where the amount of boron was insufficient to produce any blue coloration). The post-growth treated pinks were type IIa or type Ib, but with very low nitrogen concentrations.
As mentioned previously when discussing color differences in the DiamondView imaging and with color zoning, defect incorporation among the various internal growth sectors of HPHT synthetics can be quite variable. Therefore, boron and isolated nitrogen concentrations are not uniformly distributed throughout. IR absorption spectroscopy on faceted stones generally samples most of the volume of the crystal or cut stone, and the resulting spectra reflect an average concentration across the bulk of the diamond. With that caveat in mind, the electrically uncompensated boron concentration detected by IR absorption (B0) and the isolated nitrogen concentration (Ns) were calculated from 160 randomly chosen type IIb HPHT synthetics and 180 type Ib (or type IaA + type Ib) HPHT synthetics. The concentration for B0 was calculated from a method after Collins and Williams (1971) and Fisher et al. (2009), or alternatively Collins (2010). Ns was calculated from a procedure described by Kiflawi (1994). These concentration values are shown in figure 16.
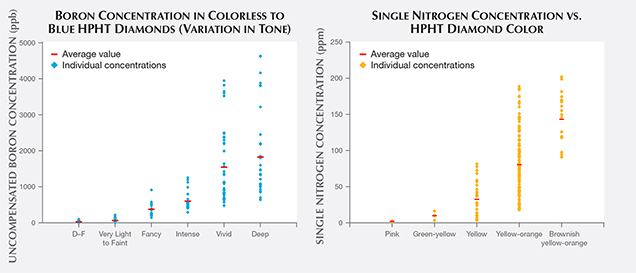
The measured boron concentrations spanned a wide range of values. Figure 16 (left) shows the calculated values for the uncompensated boron concentration, B0, in colorless HPHT synthetics and in fancy-color blues as the tone increases left to right, from Very Light up to Fancy Deep. In several cases for the Fancy Vivid and Fancy Deep samples, the boron-related feature at 2800 cm–1 in the IR spectrum was saturated (i.e., the peak height was beyond the vertical scale of the graph) and could not be calculated with this method and an alternate correlation was performed using the feature at 1290 cm–1 (Collins, 2010). The D–F colorless HPHT synthetics had the lowest boron concentrations, with the Very Light to Fancy Light blue samples encompassing a slightly higher range. The highest measured concentration was 4627 ppb, which is comparable to a value calculated for a natural type IIb diamond with very high boron (Johnson and Wang, 2015).
Figure 16 (right) shows the spread of values for the isolated nitrogen concentration for type Ib HPHT synthetics of various colors. The pinks and green-yellows had the lowest nitrogen concentrations, followed by yellow, yellow-orange, and brownish yellow-orange samples. In the early years of production in the 1980s and 1990s, most HPHT synthetics were yellow-orange, likely due to a high nitrogen concentration, which growers were not able to reduce or eliminate as effectively at the time. As the incorporation of isolated nitrogen became better controlled over the years, the orange coloration was reduced. Therefore, the manufacturers could more consistently produce HPHT synthetics with lower quantities of nitrogen, allowing a wider variety of colors and the production of more saleable stones. For example, the pink synthetics look more appealing when starting with an HPHT synthetic that has low, rather than high, concentrations of nitrogen (Shigley et al., 2004).
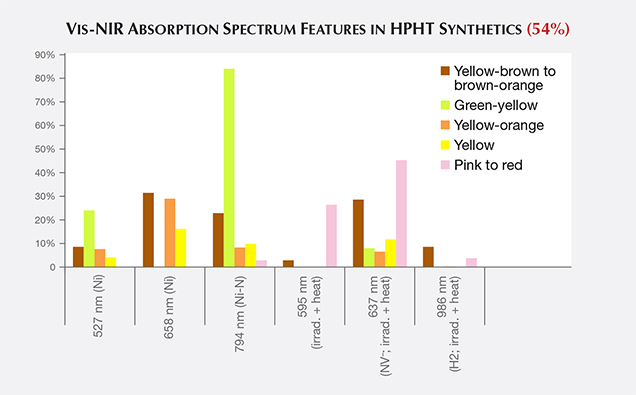
Vis-NIR Spectroscopy. For many of the fancy-color HPHT synthetics, the broadband absorptions observed in the visible/near-infrared spectra resemble those of their natural diamond counterparts. This similarity illustrates the need for multiple spectroscopic techniques and gemological observations to confirm an identification result. Spectra for blue and most yellow to yellow-orange HPHT samples are indistinguishable from natural diamond spectra. The Vis-NIR spectra of pink to red diamonds indicate the treatment history of irradiation and annealing that created the pink color rather than the synthetic origin (figure 17). For most colors of HPHT synthetics, a small percentage of the samples showed spectral peaks associated with the metal catalyst, mostly nickel (figure 17; Zaitsev, 2003).
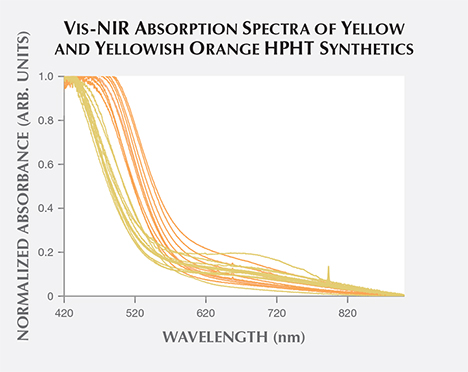
Yellow-orange HPHT synthetics comprise a majority of the samples examined by GIA to date. These include samples with color grades in order of increasing orange contribution: orangy yellow, orange-yellow, yellow-orange, and yellowish orange. To examine whether the differences in color grade between these “yellow-orange” HPHT synthetics and the pure yellow samples could be detected in the Vis-NIR spectra, we plotted 25 randomly selected yellow HPHT synthetics and 25 yellowish orange HPHT synthetics (figure 18). In these spectra, there was little difference in the absorption between the yellow and the yellowish orange synthetics at wavelengths greater than 600 nm. Below 600 nm, however, the onset of increased absorption occurs at longer wavelengths in yellowish orange synthetics. In other words, the portion of the graph showing increased absorption is shifted toward longer wavelength values for the yellowish orange samples, pushing the transmission window closer to the orange region. This absorption increase at longer wavelengths among the yellowish orange samples is due to greater nitrogen absorption within the UV range and consistent with IR calculations for the isolated nitrogen shown in figure 16 (up to 100 ppm N in yellow samples, and up to 200 ppm N in yellow-orange samples). As the HPHT synthetic process improved and less nitrogen was incorporated, the nitrogen-related absorption decreased as well.
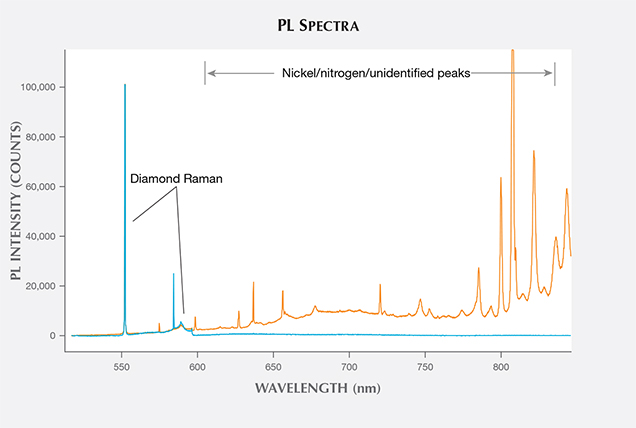
Photoluminescence (PL) Spectroscopy. PL spectroscopy is a very sensitive analytical technique that can detect light emission from optical defects at much lower concentrations than absorption spectroscopy. The PL method, where spectral features are excited by incident laser light of specific wavelengths, is therefore capable of detecting optical centers in diamond when Vis-NIR and IR absorption cannot. PL spectroscopy is a vital technique for gemological laboratories in determining diamond origin and color origin, and HPHT synthetics are no exception.
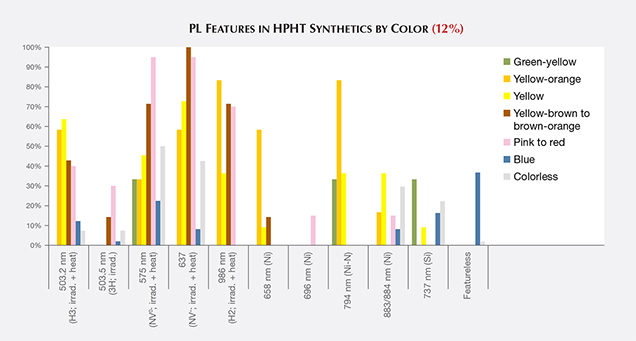
PL spectra features for HPHT synthetics were predominantly nitrogen (H3, NV0, NV–, and H2) and nickel defect-related (658, 696, 794, and 883/884 nm; Zaitsev, 2003; figure 19). While nickel was present in a notable percentage of the synthetics—the 794 nm peak was detected in 83% of the tested yellow-orange HPHT synthetics, while the 883/884 nm doublet was present in 30% of the colorless and 8% of the blues—it was not found in all synthetics and is not a reliable indicator (figure 20). It is occasionally observed in the luminescence spectra of natural diamonds (Lang et al., 2004; Eaton-Magaña et al., 2016). Not surprisingly, the HPHT synthetics with yellow coloration (including yellow, yellow-orange, and yellow-brown) had high percentages of nitrogen-related defects. Pink synthetics, while generally having a lower nitrogen concentration than the yellow synthetics, undergo post-growth coloration treatment that increases the concentration of these nitrogen-related PL defects.
There were also some spectral peaks we were unable to identify (e.g., 536, 707, 712, 912, and 953 nm; not shown), which could also correspond to nitrogen or nickel-related defects or be related to other metal catalysts or other impurities within the source materials. Also present in samples of various colors was the silicon-related defect, SiV– (737 nm; D’Haenens-Johansson et al., 2011). A significant portion of the type IIb diamonds (37%) were entirely featureless by PL spectroscopy (figure 20). Several PL features, such as 3H and peaks at 648.2 and 776.4 nm, are regularly detected in natural type IIb diamonds, yet these peaks are not observed in type IIb HPHT synthetics due to the features’ low thermal stability (Eaton-Magaña and Ardon, 2016).
FUTURE
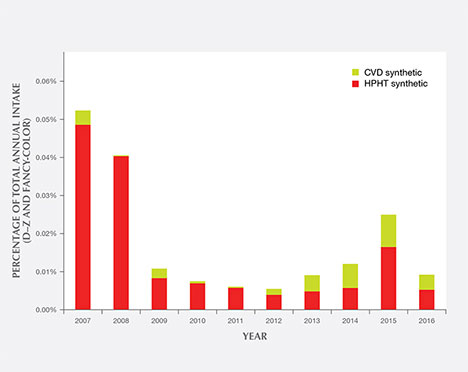
Figure 21 shows the percentages of CVD and HPHT synthetics out of the total annual intake of D–Z and fancy-color diamonds submitted to GIA’s laboratory. The overall percentages are quite small—around 0.01% in most years. The volume of synthetics was highest in 2007–2008, the first two years after the introduction of GIA’s Synthetic Diamond Grading Report, as many years of prior synthetic production were submitted. As mentioned in the Eaton-Magaña and Shigley (2016) survey of CVD synthetics, we expect that the number of HPHT synthetic diamonds submitted will continue to increase, especially at the extreme ends of the weight scale—both melee-sized and greater than 4 carats. Improvements in growth processes are making it easier to grow a large quantity of colorless melee synthetics, or to maintain growth over a long enough period to produce larger crystals.
We predict that in the coming years most HPHT synthetics will be blue or colorless, with fancy pink and fancy yellow colors available but to a lesser extent. Yellow-orange will still be submitted to GIA but in small numbers. There is already an abundance of yellow-orange HPHT synthetics, and these will be an important part of the market for many years. Other interesting products have recently been seen in GIA laboratories, such as an attractive Fancy Deep green HPHT synthetic colored by massive amounts of nickel impurities (Johnson et al., 2017). Quality factors such as carat weight, cut grade of round brilliants, and clarity will likely continue to improve over the coming years.
CONCLUSION
Today the HPHT growth process is used to produce colorless or fancy-color synthetic diamonds with high clarity. In recent years, extremes in carat weight—both melee (again, see box B) and larger sizes greater than four carats—have attracted the most attention and made the greatest strides. As growth processes continue to improve, clarity will likely improve, color zoning within fancy-color diamonds will become less noticeable, and the resulting synthetics will likely be visually less distinguishable from polished natural diamonds. Nevertheless, these HPHT synthetics display ultraviolet fluorescence reactions and inclusion features that are unusual in natural diamonds. The results described in this article illustrate how these samples all exhibit spectroscopy features that are quite different from those found in comparable natural diamonds. Based on the use of analytical instruments and access to our large database of gemological information, the synthetic diamonds examined to date by GIA can be readily identified.