Treated “Nickel-Rich” Green Diamond

Natural-color green diamonds are extremely rare. The cause of color for most green diamonds is attributed primarily to radiation damage resulting from exposure to radioactive elements over geologic time (W. Wang et al., “Natural type Ia diamond with green-yellow color due to Ni-related defects,” Fall 2007 G&G, pp. 240–243). The New York laboratory recently received a 2.12 ct pear-shaped diamond measuring 9.52 × 7.24 × 4.27 mm that was graded as Fancy yellow-green (figure 1), and testing attributed the color to several mechanisms.
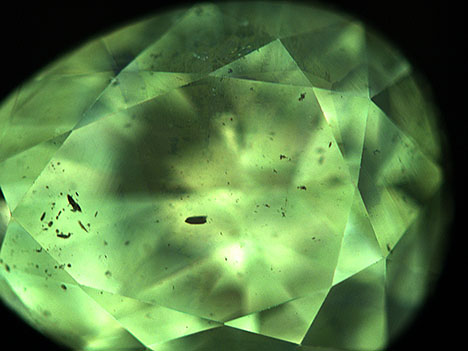
Microscopic examination revealed dark crystals and clouds throughout the stone, and infrared spectroscopy confirmed it was a type Ia diamond. DiamondView imaging showed greenish yellow fluorescence (figure 2), typical for “nickel-rich” diamond.

The ultraviolet/visible/near-infrared (UV-Vis-NIR) absorption spectrum showed a nickel-related absorption band at around 670 nm, a peak at 883 nm (an attributed peak in “nickel-rich” diamonds), and a GR1 (general radiation damage) peak at about 740 nm (figure 3). This is an uncommon combination of spectral features in diamond. The Ni-related absorption band is usually responsible for the green color in this type of diamond (Fall 2013 Lab Notes, pp. 173–174; Spring 2022 Lab Notes, pp. 50–51). In this particular diamond, the yellow-green color saturation can be attributed to both the nickel-related absorption band and the GR1 absorption. We have not observed these two features together in natural-color green diamond. Additional testing and gemological observations confirmed that the GR1 peak was artificially introduced, and we concluded that the color was treated. The color was likely a lighter and less saturated green before treatment.
This stone illustrates the importance of combining gemological observations with spectroscopic characteristics to separate natural- and treated-color green diamonds.



