Diamond Lattice Defects: GIA Researchers Explore Internal Tagging and Gray-Blue-Violet Diamond Color
February 10, 2020
GIA scientists presented their latest research on diamond tagging and the cause of gray-blue-violet color in diamonds at the Princeton-GIA Diamond Symposium, sponsored by GIA, on Jan. 24 at Princeton University.
The annual symposium is held to foster collaboration among quantum experimentalists, material scientists and industrial partners. Participants included quantum and materials research groups from the Argonne National Laboratory, Institut Néel Grenoble, City College of New York, Cornell University, CUNY College of Staten Island, IBM Quantum, Princeton and the University of Pennsylvania.
“This symposium is a wonderful way to bring together academic and industry scientists in the area that they are working on in a number of topics related to diamond, materials, spectroscopy and quantum technologies,” said Nathalie de Leon, associate professor of electrical engineering at Princeton and the organizer of the conference. “The discussions and the collaborations that resulted are incredibly valuable, and my students and I have really enjoyed the breadth of topics.”
Internal Diamond Tagging
Troy Ardon, who has a master’s in diamond science and technology from the University of Warwick in the UK and is a research associate at GIA in Carlsbad, presented "Laser Writing the H3 Defect into Natural Ia Diamond," which involves researching how to use laser writing to create an internal tagging system for diamonds.
In order to understand the possibilities, according to Ardon, you need to understand the structure of a diamond. A diamond is made up of carbon bonded together in a repeating structure known as a lattice. Pure diamond is pure carbon, nothing else, he said. It is very common, however, for non-carbon atoms (the most common is nitrogen) to be “substituted” into the diamond lattice. When a single nitrogen atom takes the place of a carbon atom, it is called substitutional nitrogen. A vacancy is just a missing carbon atom - an empty space in the lattice.
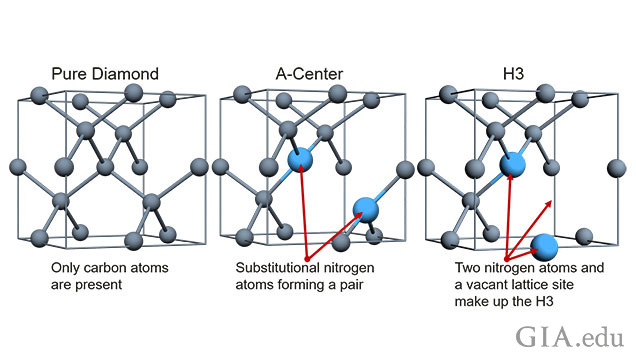
“You can combine these defects in a number of different ways, such as the H3 defect – a nitrogen-vacancy-nitrogen structure in the lattice,” Ardon said. “We used high powered focused laser pulses to knock carbon atoms out of the lattice, creating vacancies. The nitrogen was present in the form of A centers. We then annealed (slowly heated) the diamonds to ‘migrate’ the vacancies into the A-center to create the H3. We wanted to see if we could create H3 defects that can be measured above the background – one that ‘stands above the crowd’ so to speak.”
The results?
“We successfully wrote the H3 defect in a natural diamond with a low H3 background without producing visible damage,” he said. “We were not successful in writing a measurable H3 in a diamond with a high background, even with high energy pulses, which means we will have to expand our search for other writeable defects.”
Ardon said this kind of fundamental research from diamond quantum photonics ‒ a catch-all term for diamond optics and the usage of diamond defects as photon sources ‒ can be turned into a potential application for the gem industry: the creation of a robust internal tagging system. The goal is to create a unique serialized mark (similar to a QR code) to help prevent fraud and improve traceability.
“I greatly enjoy using new and fundamental research and applying it to an industry with which most people are familiar,” he said. “It almost provides a conduit to get information about high level research to a wide audience. It’s fun to connect new scientific ideas/methods with real world applications that anyone can understand.”
How do gray-to-blue-to violet diamonds get their color?
Chloe Peaker, Ph.D. and a postdoctoral research associate at GIA in New York, presented "Transition Metal Centers: A Potential Cause of Color in Gray-to-Blue-to-Violet Hydrogen-rich Diamonds," a snapshot of a theoretical and experimental study investigating what defect causes the color of rare gray-blue-violet diamonds.
“The cause of this diamond color has eluded the community thus far,” Peaker said. “The diamonds are rare and very valuable because the color is unique to naturals and has not been seen in laboratory- grown or treated stones.”
The color of the stone can be investigated by looking at the UV-visible absorption spectrum, she said, but from a theoretical perspective, identifying peaks and the responsible defect is inherently difficult.
Peaker said the study initially focused on the identification of the defect that causes the 3236 cm-1 absorption, a well-defined peak seen in Fourier-transform infrared spectroscopy (FTIR), an absorption spectroscopy technique that looks at absorption of light in the infrared range, as opposed to UV-visible spectroscopy that looks at absorption of light in the UV-visible range. The 3236 cm-1 peak is the second most prominent peak after the 3107 cm-1 peak, which was finally identified by combining theory with experimental stress results - after nearly 50 years of research.
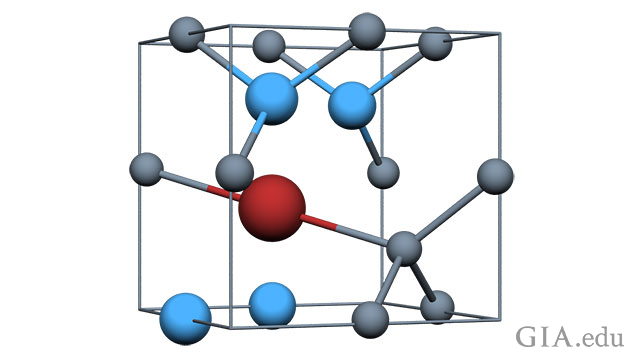
“I presented promising theoretical results of metal-nitrogen-hydrogen vibrations that are found in this region of the FTIR spectrum, although an identification based on one piece of evidence is unwise,” Peaker said. “I also speculated on the formation pathway by comparing the spatial distribution of the 3236 with the 3107 peak in mixed-habit diamonds.”
Although more work needs to be done to discover what creates the gray-to-blue-violet color in these diamonds, Peaker is eager to continue her research.
“I would love to be the one that discovers what causes the color,” she said. “Knowing what causes it would be the first step to see how the defect responsible could be reproduced or modified. I would like to find out something that could have wider implications in the diamond industry. It is exciting to research the unknown!”
The opportunity for these researchers to present at Princeton-GIA Diamond Symposium gave them access to more information about quantum theory – the study of the nature and behavior of matter and energy at the atomic and subatomic level – and its possible applications in the study of gemstones.
“The conference was well attended and all the speakers seemed to have put a lot of work into their talks,” Ardon said. “There was quite a lot of discussion about new developments in quantum computing and using diamond defects as qubits, which was fascinating to learn about.”
Peaker was impressed by what she learned about quantum computing.
“Quantum computing has grown in (quantum) leaps and bounds,” she said. “It seems like a promising future in materials science as a whole.”
Amanda J. Luke, a senior communications manager, is the editor of the GIA Insider and an editor and writer at GIA for 18 years.
.jpg)


