Decay Kinetics of Boron-Related Peak in IR Absorption of Natural Diamond
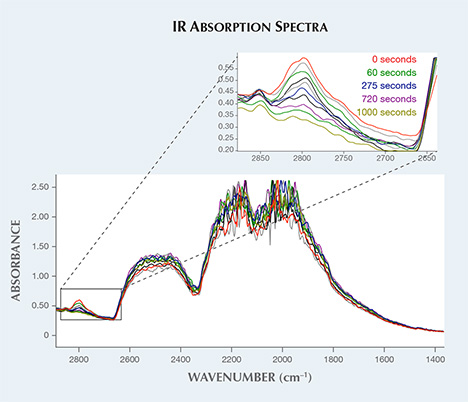
Recently, gemologists at the National Gold & Diamond Testing Center in China found that the uncompensated boron peak at 2800 cm–1 in FTIR absorption could be induced by UV excitation and then subsequent decay, similar to the phosphorescence response often seen in type IIb diamonds (J. Li et al., “A diamond with a transient 2804 cm–1 absorption peak,” Journal of Gemmology, Vol. 35, 2016, pp. 248–252).
The Carlsbad laboratory recently received a nominally type IIa, 1.01 ct diamond with Fancy gray color. It was identified as natural and, uncharacteristically, showed a 500 nm band phosphorescence, which is typical for type IIb diamonds (S. Eaton-Magaña and R. Lu, “Phosphorescence of type IIb diamonds,” Diamond and Related Materials, Vol. 20, 2011, pp. 983–989). With UV excitation we recorded the transient 2800 cm–1 absorption (associated with uncompensated boron) observed in type IIb diamonds, and we monitored the peak’s decay (figure 1).
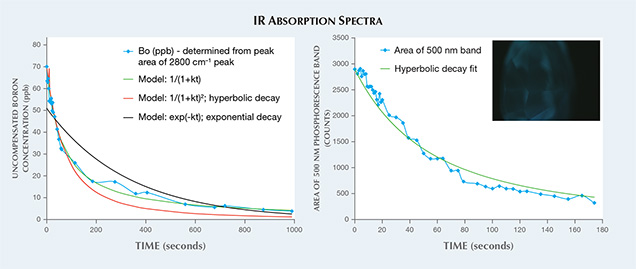
From the calculated area of the 2800 cm–1 absorption peak, we can determine the uncompensated boron (Bo) concentration (D. Fisher et al., “Brown colour in natural diamond and interaction between the brown related and other colour-inducing defects,” Journal of Physics: Condensed Matter, Vol. 21, 2009, 364213). Immediately after UV excitation, the diamond showed blue phosphorescence and the FTIR spectrum changed from a nominally type IIa diamond to a type IIb, with an uncompensated boron concentration of ~70 ppb (figure 2, left). An increase in the 2800 cm–1 peak upon UV excitation was previously recorded in some other type IIb diamonds as well and in a few other nominally type IIa diamonds examined by GIA, but those showed much lower values than this sample with UV excitation, with an initial increase in Bo of ~5 ppb or less. The decay of the 2800 cm–1 absorption band in the diamond studied here was well described by the non-exponential decay model 1/(1+kt), where t is the decay time and k is a constant. This equation fit the absorption decay data better than an exponential decay model or the hyperbolic decay model that describes type IIb diamond phosphorescence, 1/(1+kt)2 (K. Watanabe et al., “Phosphorescence in high-pressure synthetic diamond,” Diamond and Related Materials, Vol. 6, 1997, pp. 99–106).
Photoluminescence spectra were collected both with and without UV exposure using 488, 514, and 830 nm excitation. The only distinction observed between the two sets of spectra was the addition of a 3H peak (503.5 nm) when the diamond was exposed to UV radiation. The 3H peak is ascribed as an intrinsic defect containing interstitials and is often observed in PL spectra of type IIb diamonds.
Phosphorescence spectra were also recorded (figure 2, right). As expected based on prior research of diamond phosphorescence, the data did correspond well with the hyperbolic model. When boron impurities are present but are electrically compensated by other defects such as nitrogen, the 2800 cm–1 peak would not be detected and a nominally type IIa diamond would be recorded by IR absorption. However, UV excitation creates a charge transfer effect, temporarily uncompensating some of the boron so that the Bo concentration temporarily increases. This absorption decay of the 2800 cm–1 peak is most dramatic in nominally type IIa diamonds such as this sample, but has also been observed to a lesser extent in type IIb diamonds. There are several unanswered questions regarding the phosphorescence mechanism and decay kinetics in type IIb diamonds, and further study of this absorption decay will help address these issues.



