Negative Crystal Containing a Mobile CO2 Bubble in Blue Sapphire Heated with Pressure

Among the common inclusions seen in almost every mineral are primary cavities, or negative crystals. Negative crystals in sapphire are usually filled with liquid and gaseous carbon dioxide (CO2) and may also contain solids such as diaspore or graphite.
At GIA’s Bangkok laboratory, the author recently examined a 6.41 ct transparent blue faceted sapphire. It had a refractive index of 1.760–1.768, and its fluorescence reaction was inert under long- and short-wave UV radiation. Fourier-transform infrared (FTIR) spectroscopy is a useful technique to determine heat treatment in corundum. The sample’s FTIR spectrum (figure 1) showed a broad band centered around 3047 cm–1 that has been reported in sapphires treated with heat and pressure (M.S. Krzemnicki et al., “Sapphires heated with pressure – A research update,” Spring 2019 InColor, pp. 86–90).

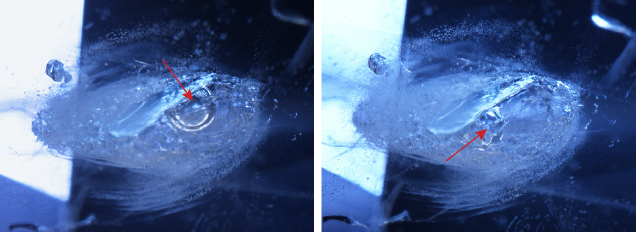
Microscopic observation revealed altered growth tubes, dissolved particles, melted crystals with altered fingerprints, and small birefringent crystals in a low-relief partially healed fissure (figure 2), proof of high-temperature heat treatment with pressure (Winter 2018 Gem News International, p. 458). When the sapphire was gently heated by the microscope bulb, the gas bubble became smaller and disappeared as the gas and liquid homogenized. The presence of CO2 fluid inclusions in negative crystals is important evidence of natural origin and the absence of thermal enhancement (J.I. Koivula, “Carbon dioxide fluid inclusions as proof of natural-colored corundum,” Fall 1986 G&G, pp. 152–155). Interestingly, this sample exhibited a rounded bubble in a negative crystal that moved and was still observed when gently heated with a hot point (figure 3). Raman spectroscopy confirmed the bubble as carbon dioxide (CO2). To our knowledge, a mobile CO2 bubble in a negative crystal has not been reported in a sapphire heated with pressure.
.jpg)


