A Fantastic Display of Phase Changes in a Sapphire’s Fluid Inclusion
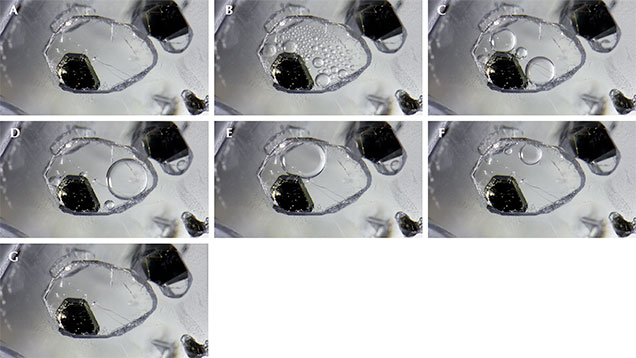
In corundum of metamorphic origin, the presence of carbon dioxide (CO2) fluid inclusions is a useful diagnostic indicator that no heat treatment has occurred; a gemologist simply has to cool the stone to below approximately 31.5°C to observe these inclusions (J.I. Koivula, “Carbon dioxide fluid inclusions as proof of natural-colored corundum,” Fall 1986 G&G, pp. 152–155). Even though this type of inclusion is considered commonplace in sapphires that form in a metamorphic environment, a spectacular example was recently witnessed in a Sri Lankan sapphire.
When viewed correctly, the inclusion is revealed to be a negative crystal with a very tabular morphology (figure 1); for more on distinguishing negative crystals, see Fall 2009 Lab Notes, pp. 212–213. The negative crystal also contains a rather large graphite crystal. As the specimen’s temperature is lowered, the CO2 trapped in the negative crystal remains homogenized with no bubbles present until reaching approximately 31.5°C, whereupon both liquid and gas phases become clearly observable. The homogenized state represents a supercritical fluid, one that behaves like a liquid and a gas under certain conditions. Under these circumstances, the CO2 assumes the shape of its “container” (the negative crystal) and can be compressed much like a gas phase while retaining the density of a liquid. This supercritical phase of CO2 has some unique properties, such as the ability to dissolve certain substances, which is otherwise impossible in its gas or liquid phase. The observation of the phase change elegantly illustrates the temperature and pressure conditions of this fluid inclusion system. Such inclusions generate pressure 75 times that of sea level (>1000 psi), and this extreme pressure accounts for their tendency to rupture during heat treatment (see E. Roedder, Fluid Inclusions, Reviews in Mineralogy, Vol. 12, Mineralogical Society of America, Washington, DC, 1984).
When CO2 is cooled below 31.5°C, the liquid and gas phases have very different densities and thus very different refractive indices, making them clearly distinguishable. Above that temperature, while in the supercritical state, the homogenized liquid has a uniform density, and the two phases are impossible to differentiate. As one raises and lowers the temperature, the changing internal scene repeatedly follows this fascinating course.
In this example of a CO2-filled negative crystal, numerous bubbles spontaneously nucleate as the temperature drops below the supercritical point. This proliferation is largely due to the flat, smooth surfaces of the tabular void. The highest-energy areas for the bubbles to nucleate exist on minute imperfections along these flat surfaces, creating an extraordinarily high nucleation density of gas bubbles. This is one of the most fantastic examples of a carbon dioxide fluid inclusion phenomenon the authors have encountered in sapphire.



