Visible Absorption Spectra of Colored Diamonds
ABSTRACT
Diamond color is usually the result of selective absorption of incident white light. The unabsorbed portion of this light is transmitted through the diamond and is interpreted by the human vision system as the perceived color. The spectroscope allows a gemologist to observe some of the more intense and narrower absorptions in the visible spectrum of diamond as dark bands at specific wavelengths. Yet the broader regions of absorption, which can be difficult to observe with the spectroscope, often have a greater influence on a diamond’s color. A chart has been prepared to illustrate the visible spectra of various colored diamonds as recorded at low (liquid-nitrogen) temperatures with a spectrophotometer. The chart shows how similar diamond colors can result from different light absorption patterns. In 2013, the authors published a simple chart listing the major optical defects that can occur at the atomic lattice level in diamond (Shigley and Breeding, 2013). The chart presented some basic information on those defects, including the ones responsible for the colors and ultraviolet fluorescence reactions of most diamonds. The brief article that accompanied the chart discussed the ongoing challenge presented by the identification of natural, treated, and synthetic diamonds. It also discussed how spectroscopy techniques, used to detect absorption and/or emission bands caused by those optical defects, play a leading role in making this important determination. GIA’s laboratory staff, which has the opportunity to examine a large number of colored diamonds, faces this identification challenge on a daily basis.
A theoretically pure and defect-free diamond would be completely colorless, and a unique attribute would be its transparency (or lack of light absorption) across a wide portion of the electromagnetic spectrum from the ultraviolet through the visible and into the infrared regions. There are, however, intrinsic absorption features in the spectra of all actual diamonds. As discussed in our 2013 article, most diamonds also contain lattice defects which, in sufficient concentrations, can produce selective absorption of incident light. (In these instances, the lattice defects are often referred to as optical defects.) When white light strikes a polished diamond, some of the light is reflected, while the rest enters the diamond where it is refracted and dispersed based on wavelength. Some of the light energies (i.e., wavelengths) are absorbed by the defects, while the unabsorbed wavelengths are transmitted. When they exit the diamond, these transmitted wavelengths in combination can create the sensation of color in the human vision system (figure 1). Visible absorption spectroscopy is the analytical tool for understanding most causes of diamond coloration.
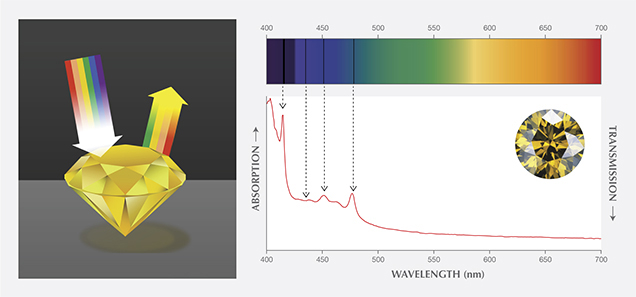
At GIA’s laboratory, visible spectra of diamonds are recorded with a spectrophotometer. Since diamond is optically isotropic, the same spectrum can be recorded in any direction through the sample. The faceted stone can be positioned in any orientation as long as the light transmitted through it is sufficient to reach the instrument’s detector. The gem’s shape and facet arrangement can make this a challenge, though. The best positions are those where the light travels directly from the table facet to the culet, or across the diamond through the opposing girdle facets. The diamond is held in a cryogenic unit and cooled to low temperatures with liquid nitrogen. Removing heat from the sample produces a less noisy spectrum. Under these conditions, absorption features that result from certain optical defects are stronger and sharper. Data collection conditions are selected to produce a spectrum that extends from about 350 to 800 nanometers (nm).
Because of a polished diamond’s high refractive index, light can be internally refracted a number of times within it, so the total distance traveled cannot be directly measured or calculated. This uncertainty makes it difficult to relate the strength of the light absorption (as recorded by the spectrophotometer) to the path length of light traveled within the gemstone. Yet this relationship can be estimated by comparing absorption peak height to the heights of some intrinsic absorption features. This relationship also provides an indirect way of comparing the visible spectra of diamonds of different sizes and faceting styles. Because of internal reflection and longer path lengths within the polished diamond, the face-up saturation of the color may appear stronger than the intensity of the absorption features (i.e., height of the absorption bands) in the visible spectrum would suggest.
Natural diamonds occur in all colors of the spectrum. A few colors, such as yellow and brown, are very common, but most are very rare. Nitrogen is the most widespread and abundant impurity element in diamond. It can be present in several optical defects, all of which produce absorption toward the blue end of the spectrum. These factors help explain the prevalence of yellow diamonds in nature. While the face-up appearance of colored diamonds is also influenced by their size and the choice of faceting style, visible absorption spectra provide a tool to understand most causes of diamond coloration.
The accompanying chart representative visible spectra and photos of the major color categories of diamond. The spectra are shown over the 400–750 nm wavelength range. Relative absorption is shown on the vertical scale of each graph, with light absorption increasing higher on the scale (and, conversely, light transmission increasing lower on the scale). Thus, the lower portion of each graph represents the transmitted portion of the spectrum that creates the material’s color sensation. The chart summarizes the major categories of colored diamonds; it is not intended as a detailed discussion of diamond coloration. The graphs are grouped in columns and ranked in descending order of frequency. The colored diamonds included in the chart were selected because their spectra (and color) are principally the result of one main lattice defect. As discussed in our 2013 article, the cause of some defects is well understood, while others are not. We occasionally encounter unusual colors in diamonds whose visible spectra do not correspond to any of the categories shown on the chart.
The information presented on the chart suggests several observations:
- In most cases, diamond colors result from different absorption spectrum patterns, with each pattern originating from one or more optical defects. This can be seen for the different yellow diamonds shown in the first column.
- Broad and intense spectral absorption features are more important in producing various colors than sharp or weak bands. Conversely, sharp absorption bands (such as the N3 observed at 415 nm, H4 at 496 nm, and H3 at 503 nm) that can be seen using the spectroscope are helpful in gem identification. We used a spectrophotometer to record the visible spectra, as it can better capture broad absorption bands that are difficult or impossible to see with the eye through a spectroscope.
- Similar diamond colors can result from different absorption spectra patterns. In other words, since perceived color is a combination of the wavelengths transmitted to the eye, different absorption patterns can produce similar colors. For example, type Ia and type Ib diamonds, which have different visible spectra patterns, can both exhibit a yellow color (although the color is usually more intense for the latter).
- Some diamond colors are the result of just one optical defect, while others result from more than one defect. For example, the chart indicates that the GR1 defect can create a blue or a green color. The defect alone produces a blue color, but when there is also absorption toward the blue end of the spectrum due to nitrogen impurities, the resulting color is green.
- Some diamond colors are simply the result of selective light absorption, while others stem from a combination of light absorption and light emission (or luminescence). For example, certain greenish yellow diamonds are yellow due to absorption, while the green component is due to luminescence.
- Variations in the types of optical defects, and in their relative concentrations, produce slight color variations among similar diamonds.
- Where a diamond photo is shown without an accompanying visible spectrum, the color is due to physical causes other than light absorption—most often the presence of mineral inclusions (e.g., numerous graphite inclusions causing black color in natural diamonds).
This chart is intended as a simple reference for those interested in understanding diamond coloration. While the visible spectra of diamonds are often presented in the gemological literature, compilations of spectra are rarely encountered.
.jpg)


