Amethyst From Morocco:
An Update

Most major sources of fine amethyst are located in Africa. Yet African amethysts on the market, especially Zambian material, tend to be dark and difficult to find in sizes larger than 10 ct. Since the late 1980s, there have been additional discoveries in Malawi, Tanzania, Namibia, Nigeria, and the Democratic Republic of Congo. The most recent African source is Morocco (see Spring 2009 GNI, pp. 62–63), which has produced gem-quality amethyst with an appealing purple color and sizes larger than 10 ct.
GIA’s Bangkok laboratory recently examined several parcels of gem-quality Moroccan amethyst (figure 1) received from Tom Banker, a colored stone dealer. Gemological properties obtained from the crystals and faceted stones were similar to those reported in the 2009 GNI entry.

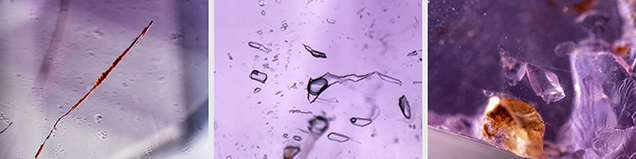
Common characteristic internal features were intense color zoning conforming to the rhombohedral faces (figure 2), reddish brown needle-like hematite inclusions (figure 3, left), and fingerprints consisting of two- and three-phase inclusions (figure 3, center). Dolomite and anhydrite inclusions were also found (figure 3, right).
A thin wafer with a thickness of 1.28 mm was prepared for FTIR and UV-Vis-NIR spectroscopy. The wafer plane was oriented parallel to the c-axis. The FTIR spectra were recorded using a Thermo Nicolet 6700 spectrometer with a resolution of 4 cm–1 and 200 scans, while the UV-Vis spectra were collected using a Hitachi U-2910 spectrometer at 1.5 nm slit width and 100 nm/min scan speed, integrated with a polarizer accessory controlled by Thorlabs APT.
FTIR spectroscopy revealed typical features of amethyst. The absorption peaks at 3585 and 3613 cm–1 were related to vibrations caused by Al substitutions. The absorption shoulder at 3595 cm–1 is common in natural quartz and rarely seen in synthetic quartz. The cause of this feature remains unknown (S. Karampelas et al., “Infrared spectroscopy of natural vs. synthetic amethyst: An update,” Fall 2011 G&G, pp. 196–201). Areas of dark, medium, and light purple zoning had very similar FTIR patterns.
Amethyst owes its purple color to color centers associated with Fe2+ or Fe3+ impurities (A.J. Cohen, “New data on the cause of smoky and amethystine color in quartz,” The Mineralogical Record, Vol. 20, No. 5, 1989, pp. 365–367). The UV-Vis-NIR absorption spectra showed bands in the visible range at 545 nm caused by Fe4+ charge transfer (E.H.M. Nunes, “Spectroscopic study of natural quartz samples,” Radiation Physics and Chemistry, Vol. 90, 2013, pp. 79–86; E. Neumann, “Mechanism of thermal conversion of color and color centers by heat treatment of amethyst,” Neues Jahrbuch für Mineralogie, Monatshefte, 1984, No. 6, pp. 272–282). The complexity of absorption bands in the ultraviolet region is related to the presence of Fe3+ in more than one environment in the α-quartz structure (F. Hassan and A.J. Cohen, “Biaxial color centers in amethyst quartz,” American Mineralogist, Vol. 59, Nos. 7–8, 1974, pp. 709–718).
Further chemical analysis using advanced techniques such as EDXRF and ICP-MS will be conducted to summarize the characteristic chemical profile of the Moroccan material.



