Gems on Canvas: Pigments Historically Sourced from Gem Materials

ABSTRACT
Pigments have been sourced from gemstones and gem materials for centuries. These colors have been applied to ancient cave drawings, classical canvas paintings, and modern artworks, revealing the evolution of the human creative experience as well as the history and progression of studies such as anthropology and chemistry. Red ochre, sourced from hematite, is one of the oldest pigments, used by early humans and even Neanderthals. Azurite was the backbone of blue pigments during the medieval and Renaissance periods. Malachite’s use as a colorant can be traced back to 6000 BCE in Egyptian cosmetics and as late as the nineteenth century in Impressionist paintings. Certain colors, such as the red of cinnabar, diminished in popularity with the invention of more affordable synthetics. Other pigments, such as bone black, continue to be manufactured and used today. The most noble of all pigments, ultramarine sourced from lapis lazuli, was so costly and valuable that only the wealthiest members of society could afford it, and its use in paintings was reserved for these patrons. Lastly, an inference can be drawn that pigment evolution correlates with the rise of significant art movements, each causing a fundamental shift in the history of art.
The wearing and collecting of gems mark their bearers with powerful symbols of status and allure. Because of their high value, gems are researched with nondestructive methods to feed growing public interest in areas such as geographic origin, synthesis, and treatment. For a gemologist, causing damage to a stone is a cardinal sin. For a painter in the past, gem materials were coveted for their pigment potential. For centuries, perfectly viable gemstones met their fate between a mortar and pestle before becoming immortalized as paint on a canvas, mural, or cave wall. These pigments commemorated color as a means of communication beyond the limits of written or spoken language. Gem materials such as hematite, azurite, malachite, lapis lazuli, bone, ivory, and cinnabar have all played roles as pigments throughout history—for some, a role assumed long before their use as gem materials (figure 1). Pigment research is an important field encompassing geologists, artists, anthropologists, historians, and even gemologists who contribute their knowledge and expertise to a subject where these disciplines converge.
Pigment can be defined as the component of paint that contributes color (Siddall, 2018). Natural inorganic pigments are derived from rocks or minerals that have been processed to extract and concentrate the material’s coloring agent (figure 2). Synthetic pigments are often chemically identical to their natural counterparts but have been produced artificially. This distinction in a pigment’s origin might seem negligible, but that is hardly the case. Synthetics strive to be chemically pure, and their crystal sizes are highly uniform. Natural pigments are never compositionally homogeneous, as rocks and minerals do not form in sterile environments. These slight imperfections in a natural pigment’s particle size and structure after processing give the color a unique fingerprint, an individualized hue that reflects light in a more complex manner than its corresponding synthetic. This property means that no two malachite greens or cinnabar reds, for example, are exactly alike—a quality that is cherished among artists. When applied to a canvas, the subtle gritty texture of natural-color paint can be seen and felt after drying, lending a more natural appearance. This quality of naturalness is similarly appreciated in gemology. Chemical impurities and physical changes that occur during a natural mineral’s formation create visual interest within the stone by means of inclusions, bodycolors, or color zoning, all of which are commonly researched and cataloged as modalities of science and art.
Binders are the second component of paint, holding the pigment particles in a concentrated suspension and then keeping the color in place after the paint has dried. Historically, binders have included natural substances such as egg yolk (tempera), linseed and poppy seed oil, tree resins, animal glues, saliva, milk, gelatin, and even blood (Carr, 2002). Even with the advent of manmade complex chemical binders, which are commonplace in acrylic paints, linseed oil and gum arabic (a hardened tree sap) are still widely used.
The study of pigment spans centuries and contributes to the greater understanding of science and art. Identifying the provenance of minerals present within pigments of a specific work relays anthropological information about the trade routes and movement of people during the period in which the piece was created. An evolution in color technology, including advances in chemical and industrial processes, can also be inferred by comparing the first cave drawings with the acrylic paintings seen in art museums today. The former consists of natural pigments such as ochres (derived from iron oxides), charcoal, and simple organic colors, while today’s paintings very often contain 100% lab-created coloring agents. The advent of affordable, mass-produced synthetic pigments is the culmination of hundreds of years of research. Prior to this revolutionary development, creating paint was expensive and highly laborious—each hue had to be mixed by hand, either by the artist or an assistant. Minerals needed for color often traveled great distances from the original source before reaching the artist, increasing the cost. The act of painting itself was reserved for those who could afford this luxury or those fortunate enough to be employed by royal families, the wealthy class, or the church. This is why most historical paintings are religious depictions or portraits of royalty and aristocrats.
The overlap of the scientific and historically artistic realms of gemstones (see box A) is a conversation not often encountered. The monetary worth and cultural significance of gems can be far inferior compared to the value of the artworks they contribute to as pigments. Hematite, azurite, malachite, lapis lazuli, bone, ivory, and cinnabar have all been significant contributors to fine art throughout history (figure 2). While most of these pigments have been replaced by synthetic equivalents, some are still used to this day.
HEMATITE
One of the earliest gemstones known to have been used as pigment is hematite. The dark, metallic color associated with gem-quality hematite is a result of densely stacked deep red microscopic crystals that ultimately absorb most visible-color wavelengths. Hematite’s red color can be seen when the mineral exists as either pulverized powder or thin crystals that allow light to pass through (figure 3). As one of the few gemstones with metallic luster, the iron oxide hematite crystallizes in the trigonal crystal system with the simple chemical formula of Fe2O3. Its height of popularity as a gem was likely in the Victorian era, when it was used extensively in mourning jewelry.

Within the realm of art, the powdered pigment form is referred to as red ochre and has been used from the dawn of artistic expression. Red ochre can also be produced by heating the mineral goethite (FeOOH, yellow ochre), most commonly sourced from limonite rock (Siddall, 2018). The use of red ochre as a pigment has been recorded in works of art from all periods and traditions around the world, from the Pleistocene to the present day (Siddall, 2018). The first use of red ochre was likely in cave paintings and as body paint. It was later used to represent blood in burial and fertility rites (Leonida, 2014), in addition to applications in sun protection, medicine, adhesives, and ceramic paint (Siddall, 2018).


The influence of red ochre on pigments is unparalleled. From the prehistoric art era (before 500 BCE) to the contemporary era, red ochre is pervasive. The earliest cave paintings from every habitable continent on Earth, revealing humans at their most primitive artistic abilities, feature red ochre. One well-known and researched example featuring this color is the cave art of Lascaux in France, dated to roughly 19,000 years ago (Musée d’Archéologie Nationale, n.d.), depicting wildlife such as bison and horses (figure 4). A younger example is the eerie Cueva de las Manos in Argentina, created 13,000 to 9,500 years ago (UNESCO World Heritage Convention, n.d.), which shows painted hand silhouettes (figure 5). It has become widely accepted by scholars that the adoption of red ochre is synonymous with the beginnings of art and therefore human intellectual evolution. In fact, the use of ochre and toolmaking are two significant advances in human evolution, with the latter universally acknowledged as an indicator of humankind’s intellectual, social, and cultural development (Wreschner et al., 1980). It can be theorized that the uniting of art and science began to take form with the use of red ochre.
The Blombos Cave archaeological site along the southern Cape coastline of South Africa has proved to be a major anthropological discovery related to red ochre. The pigment uncovered exists not as an application but as raw red ochre contained in abalone shells that slowly became buried by sands as they lay abandoned on the cave floor over thousands of years. Other materials found with the shells and ochre include cobbles, bones from seals and antelope, and stone tools. Together, these objects are believed to constitute prehistoric artistic “tool kits” dated to roughly 100,000 years old. Henshilwood and van Niekerk (2012) documented these materials and interpreted their significance: “What these findings tell us, is that the artisans who lived in Blombos Cave 100,000 years ago had the capacity for abstract thought, multi-tasking, long-term planning and an elementary knowledge of chemistry.”
Until recently it was believed that cave painting was an exclusive trait of Homo sapiens. In 2018, a team of paleoanthropologists published data on uranium-thorium dating of a series of simple drawings found inside three Spanish caves. The works examined in that study consisted of dots, lines, discs, and hand stencils, all created by red ochre (Netburn, 2018). All three were found to be at least 64,800 years old, which predates the arrival of humans in Europe by at least 20,000 years (Hoffmann et al., 2018). Neanderthals exclusively populated this region of modern-day Europe at the time, implying that the artists were indeed Neanderthals. Proof of their ability to create art helps dispel the popular misconception that Neanderthals were mentally inferior to Homo sapiens.

Nearly every canonized artist has used red ochre at some point. The color was also a traditional ingredient in sanguine, a type of chalk colored by red ochre (figure 6). Leonardo da Vinci grew fond of the material, featuring it in numerous drawings during the Renaissance period. Da Vinci is credited with being the first major artist to use this ochre variety (Millidge, 2003), and Michelangelo continued its reach. This period also popularized the use of red ochre in fresco murals. Paul Gauguin, one of the most famous painters of the Post-Impressionist movement, made it a staple of his palette. Red ochre’s importance to modern artworks is incalculable. Twentieth-century masters such as Pablo Picasso, Mark Rothko, and Andy Warhol created works featuring the color, bringing it full circle. While most natural pigments have been far surpassed by synthetic pigments, red ochre is the exception. Red ochre paints continue to be predominantly made with natural hematite or heated goethite due to the abundance and low cost of these materials.
AZURITE AND MALACHITE
Malachite, Cu2(CO3)(OH)2, is perhaps the first vivid green pigment (Bergslien, 2012). A basic carbonate of copper, it is the weathered form of the blue parent mineral azurite, (Cu23+(CO3)2(OH)2), and possesses a similar chemical formula. Azurite and malachite are rarely found exclusive from one another and form in exposed areas of copper ore. Both minerals have a monoclinic crystal structure and a low Mohs hardness of 3.5–4.0. Malachite has been used extensively as a decorative material since antiquity. Azurite’s decorative use has been far more limited by its low durability and high likelihood of breaking along cleavage planes. It has mainly been reserved for pigments.
Possibly the earliest application of azurite and malachite came in the form of cosmetics. Malachite pigment can be traced to ancient Egypt, where it was used as an eye paint during the predynastic period, spanning from 6000 BCE to 3100 BCE (Gettens and FitzHugh, 1993b). Likewise, high-purity, coarsely ground azurite particles have been traced to Neolithic female and infant burial sites at the Central Anatolian site of Çatalhöyük (modern-day Turkey) and dated to 6700 BCE (Siddall, 2018), where the mineral was also likely used as a cosmetic material. The same era saw both minerals used in the Middle East to color soapstone ornaments, beginning around 4500 BCE (Ball, 2002).
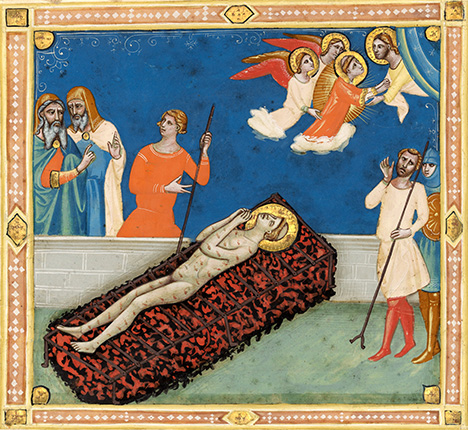
Although azurite is far less abundant than malachite, azurite pigment has been utilized more widely. It was the most important blue pigment in Europe throughout the medieval period (figure 7) and enjoyed peak use during the Renaissance (Gettens and FitzHugh, 1993a). This is because of its dual role as not only a royal color but also as an underpaint for the lavish ultramarine (a pigment from lapis lazuli). Both pigments were used for centuries in Japan, in Ukiyo-e style paintings (sixteenth through nineteenth centuries; Gettens and FitzHugh, 1993a,b) and malachite in screen and scroll paintings to the present day (figure 8). Historical Chinese artworks also feature the two extensively, spanning hundreds of years.
Copper and copper-containing metals are most commonly associated with bright green patina, a color and pigment material known as verdigris. Chinese history reveals one cunning application of malachite in imitation of verdigris. Beginning around 1000 CE, patina—the surface discoloration of certain metals from long periods of oxidation—became associated with ancient bronzes unearthed in China (Craddock, 2003). This feature became a sought-after trait among antique bronze collectors, offering a sense of authenticity. Bronze statues excavated and collected during the Song (960–1279 CE), Ming (1368–1644 CE), and Qing (1644–1911 CE) dynasties were often imitated by carefully painting replicas with malachite pigment to achieve a faux patina effect. Blue azurite patina is less prevalent but still possible in specific conditions.
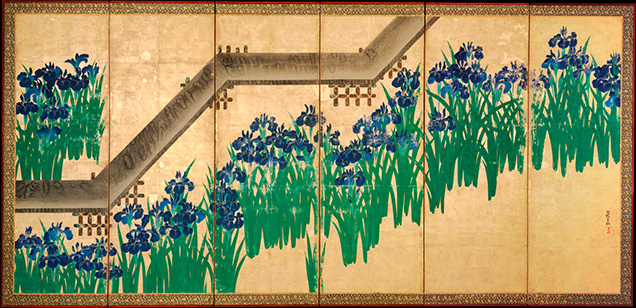
In European easel painting, malachite was vitally important from the fourteenth to seventeenth centuries, until the rise of copper greens such as verdigris and copper resinate (Eastaugh et al., 2004). Synthetic green pigments replaced malachite around 1800 (Bergslien, 2012). Malachite experienced a brief resurgence later in the nineteenth century, and it was during this period that Pierre-Auguste Renoir painted Chrysanthemums (figure 9). Renoir helped solidify the Impressionist movement along with contemporaries such as Monet, Cézanne, Degas, and Manet. Impressionism is distinguished by short, coarse brushstrokes creating a spontaneous unfinished appearance, vibrant color palettes, and themes of nature. The movement was bolstered by readily available premade oil paints in tubes (Newman et al., 2019). This brought unprecedented mobility, allowing pioneering artists to take their work outdoors. The movement thrived from the mid- to late nineteenth century and is considered the most important influence on modern art, since it did not follow established conventions.
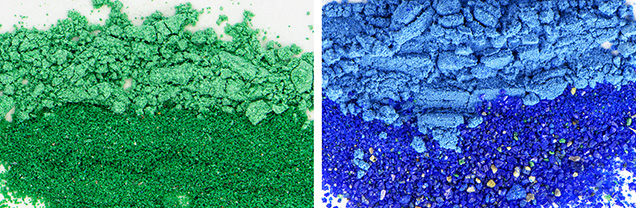
One inconvenience drove malachite and azurite into obscurity as pigments: the fact that their color is dependent on particle size (figure 10). Finely ground material offers a preferable texture for canvases but reduces the color to an undesirable milky pastel. Coarse particles offer a radiant hue but are difficult to paint in layers. Azurite became obsolete in the nineteenth century with the invention of the artificial pigment Prussian blue (Gettens and FitzHugh, 1993a).
LAPIS LAZULI
Lapis lazuli is a complex metamorphic rock consisting of a variety of minerals, often including calcite, pyrite, diopside, amphibole, and feldspathoid silicates, among others. The blue color source within lapis remains an open question. Lazurite is commonly believed to be the blue constituent, but several publications have credited the mineral haüyne. The two minerals, along with sodalite and nosean, are members of the sodalite group. Several studies have shown that haüyne (sulfate member) rather than lazurite (sulfide member) is consistently the dominant species in lapis from Sar-e-Sang in Afghanistan and Baffin Island in Canada (Hassan et al., 1985; Fleet et al., 2005; Moore and Woodside, 2014). Meanwhile, specimens from the Coquimbo region of Chile have been characterized as lazurite-dominant (Coenraads et al., 2000).
Of all the natural pigments created throughout history, ultramarine, a blue derived from lapis lazuli, reigned supreme. Cennino Cennini was a fifteenth-century Italian painter and author of The Craftsman’s Handbook (1437), an artist’s manual on methods and techniques that remains remarkably relevant today. Cennini held the pigment in the highest regard: “Ultramarine blue is a color illustrious, beautiful, and most perfect, beyond all colors; one could not say anything about it, or do anything with it, that its quality would not still surpass.” During its prime, the blue was deemed so sacred that it was reserved for the most important works and only the holiest of religious figures. Ultramarine was said to be as expensive as an equal weight of gold. Its high cost was due to the inconvenience of only one source location supported by the arduous procedure required to process the rock into pure pigment. Lapis lazuli’s life as a pigment can be traced to the origins of human civilization itself.
While blue seems abundant in nature given the color of the sky and sea and other smaller examples, none of these actually contain a blue pigment. Instead, the blue color of the sky is a result of light scattering off the molecules in the atmosphere, called Rayleigh scattering. Sea water is blue due to the preferential absorption of long-wavelength (red) light. The cause of blue in both cases is a result of light physics rather than chemical properties. Only a handful of plants and animals possess a genuine blue pigment. This left few options for artists of the past. Azurite was predominant from the medieval period to the Renaissance (Plesters, 1993). Its limitation is a typically green undertone that cannot be removed. In contrast, ultramarine often contains a violet to purplish undertone (figure 2), creating an unequivocal color that came to be associated with divinity.

Due to its geological rarity, lapis lazuli sourced in antiquity originated from a single location—the Sar-e-Sang mines in the Badakhshan Mountains of northeastern Afghanistan (Siddall, 2018) (figure 11). Lapis mining at Sar-e-Sang began in the Stone Age, with lapis jewelry found in graves of the Mehrgarh people (a Neolithic settlement located in present-day southwest Pakistan) dated to 7000 BCE.
Lapis was exported to the ancient Sumer civilization of Mesopotamia around 3000 BCE before arriving in Egypt during predynastic times and becoming prevalent by the First Dynasty (ca. 3100–2900 BCE) (Moore and Woodside, 2014). Egyptians utilized lapis in jewelry and decorative inlays, medicinal preparations, and cosmetic pigments. Perhaps the most famous artifact of ancient Egypt, the funerary mask of the pharaoh Tutankhamun, features a variety of inlaid gems: obsidian, white quartz, lapis lazuli, turquoise, amazonite, carnelian, and other stones (Reeves, 2015). A portion of the lapis inlay serves as Tutankhamun’s eyeliner, a representation of the cosmetic pigment worn by the elite.

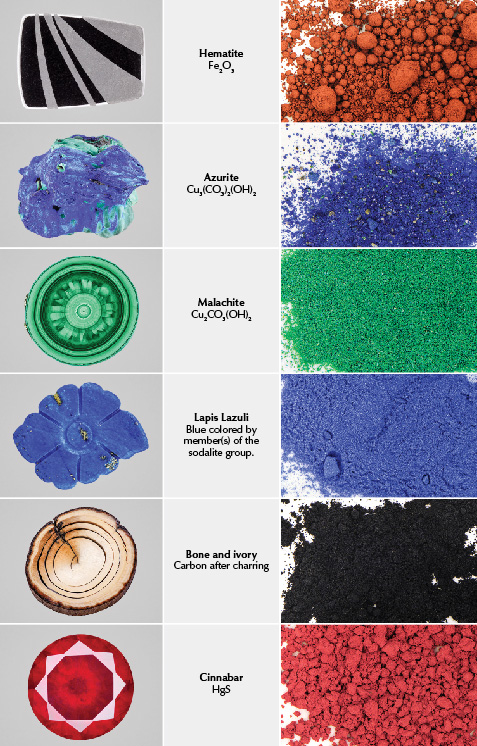
The earliest discovery of ultramarine was in oil paintings on cave walls in Bamiyan, Afghanistan, likely created in the late sixth century and consisting of Buddhist subjects drawn in semi-Indian, semi-Persian style (Gettens, 1938). Significantly, this finding also revealed the oldest known use of oil as a binder (Cotte et al., 2008). Ultramarine emerged in Europe during the early medieval period (Siddall, 2018) and rose in popularity into the fourteenth to mid-fifteenth centuries, when it was used heavily in panel paintings and illuminated manuscripts (Plesters, 1993). In paintings from the fourteenth to sixteenth centuries, the highest-quality ultramarine was reserved for the cloaks of Christ and the Virgin Mary (Plesters, 1993) (figure 12). Ultramarine was the most expensive pigment during its prime, meaning it could only be used sparingly (Plesters, 1993).


Ultramarine’s popularity was bolstered by the mass publication of the process required to extract pigment from lapis lazuli. Because lapis lazuli is a rock containing various accessory minerals such as calcite and pyrite, the measures used in the preparation of azurite such as simple milling, washing, and sieving were not sufficient (Plesters, 1993). The accessory minerals, especially pyrite, would darken and discolor ultramarine’s brilliant blue if not extracted. Cennini documented the extraction process in The Craftsman’s Handbook, and his method is still used today. It involves repeatedly crushing and sieving the highest-quality lapis. The powder is then worked into a dough with various waxes and kneaded under a liquid solution of lye. The fine blue particles slowly precipitate out of the dough and into the solution, while heavier materials such as pyrite are retained. Once the liquid has evaporated, one is left with ultramarine. The process can take up to several months—another factor influencing its high cost. The ensuing centuries saw ultramarine blue ascend to unequaled prestige in Europe, and works featuring the color went on to achieve everlasting fame (figures 13 and 14).

While ultramarine most often appeared in Christian art, it was sometimes used to create a picturesque sky on canvas. The sky’s features mimicked those of lapis lazuli, whose scintillating pyrite and globules of calcite in a sea of deep blue resemble stars and clouds. This hallmark is seen in Bacchus and Ariadne (figure 15), one of Titian’s most famous works, which also incorporates azurite, malachite, and cinnabar.
For a color as fabled as ultramarine, it is only fitting that its decline would also be chronicled. In 1824, France’s Society for the Encouragement of National Industry announced a contest for synthesizing artificial ultramarine, with a prize of 6,000 francs (Plesters, 1993). Four years later, a process was discovered and Jean Baptiste Guimet was named the winner. This synthetic, often referred to as “French ultramarine,” sold for approximately one-tenth the cost of the natural material. In the mid-nineteenth century, it was manufactured throughout Europe and quickly outsold the natural variant, as it still does today.
BONE AND IVORY
For centuries, bone and ivory have been used to create black pigments. These materials are complex, consisting of both organic and inorganic compounds. Most recent studies have identified the composition as carbonate hydroxylapatite (Eastaugh et al., 2004). When bone or ivory is heated in the absence of oxygen via a closed crucible, black pigment is produced. The carbon source is primarily the protein collagen (Winter and FitzHugh, 2007), which is incorporated in the matrix of the material.
Bone is one of the oldest known gem materials. Recently, archaeologists discovered a more than 46,000-year-old aboriginal nose ring made of bone at a site in Western Australia, the oldest bone implement found on the continent (Langley et al., 2016). A variety of animal bones have been used as pigment source material throughout history, including cattle and lamb and possibly even human remains in earlier centuries (Finlay, 2002). Documents from the current largest producer of bone black pigment in the United States, Ebonex Corporation, specify charcoaled cow bone as their source material (Ebonex Corporation Activity Report, 2018).

The Roman naturalist Pliny the Elder credited the development of ivory black to Apelles, the most notable painter of ancient Greece, though none of his works have survived (Pliny, 77 CE). As the name implies, genuine ivory black was created from ivory wastes. These wastes were in relative abundance from the fifteenth to nineteenth centuries, as ivory was widely traded throughout many parts of the world. It was fashioned into jewelry, tools, weapons, containers, musical instruments, billiard balls, and other novelties (Smithsonian National Museum of African Art, 2019). Ivory black pigment was manufactured until the end of World War II (Kremer Pigmente, 1985). Due to species protection measures, all ivory black sold on the market today must be sourced solely from old stock (Kremer Pigmente, 1985) or be composed of high-grade bone black (Winter and FitzHugh, 2007). Ivory black is reportedly more intensely black than bone black, but this is arguably due to the pigment simply being more carefully made, since ivory has always been more scarce than bone (Winter and FitzHugh, 2007). Ivory was also used for paint palettes in previous centuries (figure 16).

Bone black has been found in prehistoric, Egyptian, Greek, Roman, medieval, and Renaissance art (Coles, 2018). Optical microscopy revealed its use on gravestones in ancient Greece from the third to second century (Winter and FitzHugh, 2007). Ivory and bone black have been scientifically identified in western European art dating from at least the fifteenth century into the twentieth century, including works by Tintoretto, Rubens, Rembrandt, Manet, and Renoir (Winter and FitzHugh, 2007). An extensive analysis of works in the Museum of Pablo Picasso in Paris concluded that 62 of his paintings contained either ivory or bone black (Winter and FitzHugh, 2007). Modern artist Kazimir Malevich, who founded the Suprematism movement and (along with Picasso) helped propel and popularize abstract art, featured ivory black in his signature geometric style (figure 17).
The process for creating white pigment from bone can be duplicated in the presence of oxygen. Essentially the ash that remains after all organic material has been destroyed, bone white was first used in the Neolithic period (Coles, 2018), primarily as a paper preparation in metalpoint drawing. In this technique, a soft metal writing utensil (silver, gold, or copper) grazes across a paper that has been primed, usually with bone white pigment mixed with rabbit skin glue. Bone ash possesses a slightly abrasive quality, allowing the metal to exfoliate and adhere to the primed surface, similar to a modern graphite pencil against paper. Graphite eventually became more popular because it was easier to use, causing metalpoint to fade into obscurity. While bone ash has little use as a modern-day pigment, bone black is still sold at art stores.
CINNABAR
Cinnabar (HgS) is an intensely colored mercury sulfide mineral and the principal ore of mercury. It is not abundant in the earth’s crust, and only a handful of important deposits occur in Europe, the Middle East, and Asia. It is usually found with a massive habit, though well-formed, gem-quality single crystals are occasionally discovered (figure 2). Its hue is a strikingly vivid red with a strong orange component, unlike the comparatively dark and common red ochre. In its simplest form, cinnabar’s color is derived from simply crushing and grinding the mineral in a stone mortar. A synthetic form, commonly referred to as vermilion, has existed for several centuries and is obtained from synthesizing mercury and sulfur. After ultramarine, cinnabar was historically the most valuable and prestigious pigment in the trade, with Spanish and Chinese sources being the most significant (Siddall, 2018).

One of the earliest uses of cinnabar as pigment was in the Middle East in Çatalhöyük, an early human settlement from 7100 BCE to 5700 BCE, where it has been found in paintings and burial contexts (Çamurcuoğlu, 2015). Of the more than 800 Dead Sea Scrolls discovered in Israel and considered the world’s earliest copies of biblical books, four fragments have been shown to contain red ink composed of cinnabar (Nir-El and Broshi, 1996). Ancient Romans indulged in the use of the pigment in wall paintings, assigning it great importance and sacred associations (Spindler, 2018) (figure 18). Pure cinnabar pigment could turn black when exposed to light, prompting the Roman scholars Vitruvius and Pliny the Elder to use a coating of oil or wax in their work (Eastaugh et al., 2004). Recent studies have shown that this discoloration is actually associated with cinnabar that has either been exposed to halogen or contains traces of chlorine (Eastaugh et al., 2004).
Cinnabar also experienced a widespread cultural diffusion in China. During the Shang and Zhou dynasties (1600–256 BCE), it was used for strewing of remains in grave burials, presumably to preserve the dead (Gettens et al., 1993). Treasured in Chinese alchemy, cinnabar was an important ingredient in recipes for preparing the philosopher’s stone (a mythical substance believed to turn common metals into gold) and medieval pharmaceutical elixirs (Gettens et al., 1993). Traditional Chinese medicine prescribed powdered cinnabar to treat a variety of medical conditions including skin infections and intestinal disorders (Liu et al., 2008). Many of these cinnabar remedies are still used in Chinese medicine.

Cinnabar pigment was used considerably in Chinese lacquerware—a material that dates to 7000 BCE and is still produced today (Siddall, 2018). Lacquer is a resin primarily sourced from the tree species Toxicodendron vernicifluum. When exposed to oxygen and dried, it transforms into a natural plastic that is resistant to heat and water. Lacquerware is created from a base of turned wood with 30 to 200 layers of lacquer applied (Metropolitan Museum of Art, 2009). Once hardened, lacquer can be elaborately carved into geometric motifs or extraordinary representations of earth, water, or sky (figure 19). These items were most often colored red and became known as “cinnabar lacquer.”
Vermilion is an artificially created cinnabar that can be produced through either a wet or dry process. The dry process may have been invented in China before spreading west through Arab traders, with the first documentation of this process originating in the eighth century (Gettens et al., 1993). Medieval recipes for dry-process vermilion involve combining mercury with molten sulfur and heating until the compound sublimes and condenses. The final product is a red crystalline modification of mercury sulfide. It is then treated with an alkali solution to remove free sulfur, washed, and ground under water in preparation as pigment. The wet process, discovered in the seventeenth century, calls for the combination of mercury sulfide and a heated solution of ammonium or potassium sulfide. This process was more cost efficient and became the favored method of vermilion production in the West. Obscure in the eighth century, vermilion had become mainstream by the fourteenth century (Gettens et al., 1993). Unlike malachite and azurite, cinnabar and vermilion are strong light absorbers whose colors are preserved at all particle sizes.

Vermilion was an important color in illuminated manuscripts as it was used to paint the rubricae (text written/printed in red ink for emphasis) and imagery. It became a staple from the fourteenth century onward (Gettens et al., 1993), appearing in the works of Vermeer (figure 14), Titian (figure 15), and Degas (figure 20). In the early nineteenth century, cadmium red was introduced and began to take the place of vermilion (Melo and Miguel, 2010), which had already surpassed cinnabar in production and usage. Cadmium pigments have since become the standard for brilliant, lightfast, and weather-resistant yellow, red, and orange paints.
CONCLUSIONS
Many historical pigments led double lives as gem materials, and both commodities have retained value over time. While the beauty of gems is appreciated by society, art viewers are often unaware that acclaimed paintings from the prehistoric to postmodern eras feature colors obtained from ornamental materials. Pigments and gems are further intertwined by their ability to reveal anthropological information about humankind’s comprehension of the natural world. With a better understanding of chemistry, the synthesis of both pigments and gemstones inevitably followed. The availability of a variety of gem materials shaped the development of pigments, which in turn shaped the history of art. As art is merely a psychological reflection of and reaction to our environment, the conversation of art has contributed to the shaping of humankind itself.

.jpg)


