Grossular Garnet Crystals in Demantoid Garnet

Demantoid, the green variety of andradite garnet, is a rare gemstone. It typically possesses high dispersion and diamond-like luster, which is often partly masked by the green and yellow-green bodycolors. Variations in the color of demantoid garnet are generally caused by traces of chromium (Cr3+), ferric iron (Fe3+), Fe2+-Ti4+ intervalence charge transfer, and Fe2+-Fe3+ interactions (G. Giuliani et al., “Gem andradite garnet deposits demantoid variety,” InColor, No. 36, Summer 2017, pp. 28–39).
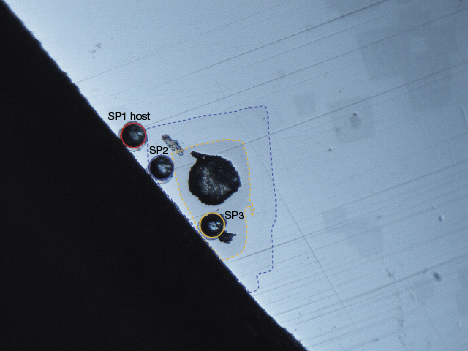
Recently, GIA’s Bangkok laboratory received a 4.47 ct yellowish green gemstone for identification. Standard gemological testing, including a refractive index over the limits of the refractometer and a specific gravity of 3.86, and Raman spectroscopy identified it as demantoid garnet. Microscopic examination revealed strong internal graining, fingerprints, and particles mixed with short needles. Interestingly, it also exhibited transparent octahedral crystals at the crown (figure 1). Some of them reached the surface and had a different luster from the host material. Close examination showed that the crystals contained two different luster areas, which also distinguished them from the host demantoid (figures 2 and 3).

Raman spectroscopy was performed on the three different areas to identify the materials. The Raman spectra of the two areas of the crystals were similar to that of the host, matching with andradite garnet. Garnet species typically possess similar physical properties and crystal forms, but differ in chemical composition. For example, andradite is a calcium-iron garnet with the formula Ca3Fe2(SiO4)3, whereas grossular is a calcium-aluminum garnet with the formula Ca3Al2(SiO4)3. Those two garnet species are in the same isomorphous series and therefore have the same general chemical formula, but differ only in containing Fe or Al. Due to their similar Raman spectra, chemical analysis is better suited to separate the species of garnet.
In this case, laser ablation–inductively coupled plasma–mass spectrometry (LA-ICP-MS) was used to compare the chemical composition of the demantoid against that of the surface-reaching inclusions. Spot 1 was on the host material, spot 2 on the outer area of the crystal inclusion, and spot 3 on the inner area of the crystal (figure 3).

Surprisingly, the three spot areas exhibited different chemical compositions, especially between the host demantoid area and the inner area of the crystal. This indicated that the crystal inclusion was from a garnet species other than andradite. Table 1 shows the chemical composition of the three spot areas, provided first in parts per million by weight (ppmw) units, followed by wt.% oxides of elements, and then as recalculated cations normalized to 12 oxygen atoms. Spot 1 (the demantoid) contained significantly more Fe than Al, whereas spots 2 and 3 of the crystal inclusion contained both elements. Spot 2 contained a higher concentration of Fe than Al, and the calculation showed a mix of two garnet species. It is possible that the detection area partly included the host material. Spot 3 contained a higher Al concentration than Fe (a 75.36% match with grossular).
Many types of solid inclusions are found in demantoid garnet, such as chromite, apatite, and diopside. From the results obtained here, it is interesting that solid inclusions of a different garnet species—grossular, in this case—can also be trapped in demantoid.
.jpg)


