Aquamarine from Pakistan

In recent decades, a steady supply of aquamarine has come from northern Pakistan, an increasingly important mining locality. The aquamarine was mined mostly from the Gilgit-Baltistan region, including the Shigar Valley, as well as the Hunza and Braldu Valleys (figure 1). The aquamarine occurs in zoned pegmatites formed by hydrothermal fluids in the cavities or veins (M.H. Agheem et al., “Shigar Valley gemstones, their chemical composition and origin, Skardu, Gilgit-Baltistan, Pakistan,” Arabian Journal of Geosciences, Vol. 7, No. 9, 2014, pp. 3801–3814).

Most aquamarines from northern Pakistan show a long hexagonal prism habit and can reach up to a dozen centimeters in length (figure 2). Albite, muscovite, and tourmaline (schorl) are commonly associated as matrix, and most of these are collected as mineral specimens. Parts of them are gem quality and suitable for jewelry use.
Aquamarine samples from the Shigar Valley were obtained from local merchants who deal with Pakistani material. The samples displayed colors ranging from pale greenish blue and pale blue to nearly colorless, with a translucent to transparent appearance. The samples measured 1–2 cm in length and 0.5–1 cm in diameter. To explore the gemological and other properties of these samples, six of them (figure 3) were investigated by standard gemological methods as well as spectroscopic and chemical analyses at China University of Geosciences (Wuhan).

The following gemological properties were recorded: refractive index (RI)—no = 1.574–1.580, ne = 1.569–1.575; birefringence—0.005–0.006; specific gravity (SG)—2.60–2.72; UV fluorescence—weak bluish white to both long- and short-wave UV. The moderate to weak dichroism observed was a relatively saturated blue or greenish blue along the e-ray and very pale blue, greenish blue, or near-colorless along the o-ray.
Microscopic observation revealed abundant 20–300 µm inclusions, either two-phase (fluid, gas) or three-phase (one or two types of fluid, solid, gas) with various shapes. The irregularly shaped multiphase inclusions often gathered together to form a complex “fingerprint” network distributed along healed fissure planes (figure 4, top row). In addition, two-phase inclusions with elongated shapes parallel to the c-axis, and with hexagonal outer profiles perpendicular to the c-axis, were consistent with the crystalline symmetry of beryl (figure 4, bottom row).
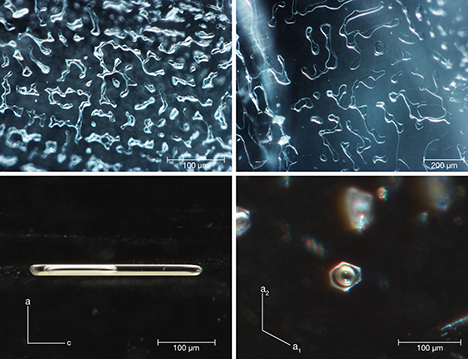
These two kinds of multiphase inclusions formed by different crystallization processes. When the beryl crystallized out of a hydrothermal fluid, some of the fluid was trapped. With dropping temperature during mineralization, beryl was re-deposited and a gaseous phase was released from the fluid, gradually forming single multiphase inclusions whose shape conformed to beryl’s hexagonal symmetry. But the formation of multiphase “fingerprint-like” inclusions with irregular shape occurred later. Fluid was embedded in the fracture of the beryl crystal. Due to the solution and re-deposition of beryl from fluid on the fracture surfaces, a small amount of liquid and released gas were gathered and closed off, just like “fingerprint” or dendritic growth networks along healed fissure planes. For a detailed description of these processes, please refer to E. Roedder, “Ancient fluids in crystals,” Scientific American, Vol. 207, No. 4, 1962, pp. 38–47.
Large amounts of multiphase inclusions could lead to a misty, translucent, and uneven appearance, such as the aquamarine crystal in figure 2, with a misty translucence toward the bedrock end. Generally, these characteristics can also be seen in aquamarine from other deposits in Brazil, the United States, South Africa, and China (D. Belakovskiy et al., Beryl and Its Color Varieties: Aquamarine, Heliodor, Morganite, Goshenite, Emerald, and Red Beryl, Lapis International LLC, East Hampton, Connecticut, 2005).
Multiphase and mineral inclusions were identified by Raman spectroscopy with 532 nm laser excitation. The gaseous phase was identified as a mixture of CO2 and a small amount of methane (CH4), and the fluid inclusion consisted of water with dissolved CO2 or liquid CO2. Solid phases inside the multiphase inclusions were identified as solid sulfur and orpiment. Along with columnar tourmaline, white albite, and flaky muscovite, other mineral inclusions such as orange-red almandine, brown tantalite, and yellow argentojarosite [AgFe3(SO4)2(OH)6] were identified by Raman.
Chemical analysis on aquamarine samples by laser ablation–inductively coupled plasma–mass spectrometry (LA-ICP-MS) showed the following ranges for alkali elements: Li 70–1240 ppmw, Na 830–3970 ppmw, K 54–453 ppmw, Rb 10–64 ppmw, and Cs 310–3220 ppmw. The amount of total alkali content including Li, Na, K, Rb, and Cs ranged from 1296 to 6033 ppmw. As alkali ions (mainly for Na) were associated with “type II” water molecules in structural channels (D.L. Wood and K. Nassau, “The characterization of beryl and emerald by visible and infrared absorption spectroscopy,” American Mineralogist, Vol. 53, No. 5, 1968, pp. 777–800), the relatively low Na content was in agreement with the low peak intensity of “type II” H2O revealed by Raman and IR spectroscopy. The low concentration of alkali content and lack of “type II” water in our aquamarine samples were similar to that in aquamarine from Italy and Vietnam (R. Bocchio et al., “Aquamarine from the Masino-Bregaglia Massif, Central Alps, Italy,” Fall 2009 G&G, pp. 204–207; L.T.-T. Huong et al., “Aquamarine from the Thuong Xuan District, Thanh Hoa Province, Vietnam,” Spring 2011 G&G, pp. 42–48). The chromophore Fe, responsible for blue color, was the richest among all transition elements between 1350 and 5080 ppmw; the samples showed the typical iron absorption at 375, 425, 620, and 820 nm in UV-Vis-NIR spectra. Transition elements V and Mn were present at 1–33 ppmw and 17–56 ppmw, respectively, and the bluish white fluorescence mentioned above may originate from Mn2+ or VO4 centers (M. Gaft et al., Modern Luminescence Spectroscopy of Minerals and Materials, 2005, Springer Berlin, pp. 97–99). Chemical and spectral characteristic suggested the Pakistani samples were low-alkali aquamarine colored by Fe ions rather than irradiation (i.e., Maxixe beryl).



