Impregnated Amazonite

A type of high-quality translucent blue amazonite feldspar (figure 1) has recently appeared in the jewelry market. Its vivid blue color and unusually high translucency are rarely seen in untreated mineral specimens and rough materials. A rough piece of this material and five beads with different quality grades were obtained for detailed examination at GIA’s Carlsbad laboratory.
To induce the blue-green color in amazonite (KAlSi3O8), the potassium feldspar must contain structurally bound water in addition to lead, and then undergo irradiation (A.M. Hofmeister and G.R. Rossman, “A spectroscopic study of irradiation coloring of amazonite: structurally hydrous, Pb-bearing feldspar,” American Mineralogist, Vol. 70, 1985, pp. 794–804).
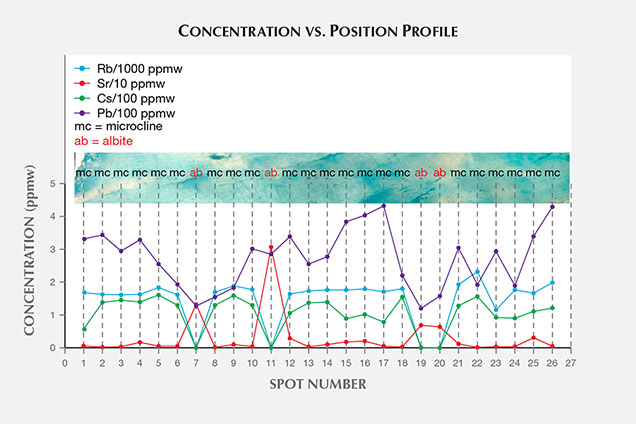
FTIR spectroscopy revealed resin impregnation. A thin section cut from the middle of the rough (top of figure 2) was prepared to investigate the depth of impregnation and the origin of the vivid blue color. By performing FTIR in the middle of the section, we confirmed that the impregnation completely penetrated the material. Chemical analysis was obtained with a Thermo Fisher iCAP Q ICP-MS coupled with a New Wave Research UP-213 nm laser ablation unit. A line of 27 ablation spots crossed the whole section. Analysis revealed a microcline feldspar composition with a very minor albite component (figure 2). Pb concentration was irregularly distributed from one rim to the other, eliminating the possibility of Pb diffusion treatment (figure 2, violet data set). The concentration range of Pb was 11.7–44.6 ppma, or 120–432 ppmw. Albite portions contained higher Sr (red data set) and no Rb (blue data set) or Cs (green data set). In contrast, microcline portions contained higher Rb and Cs, and almost no Sr.
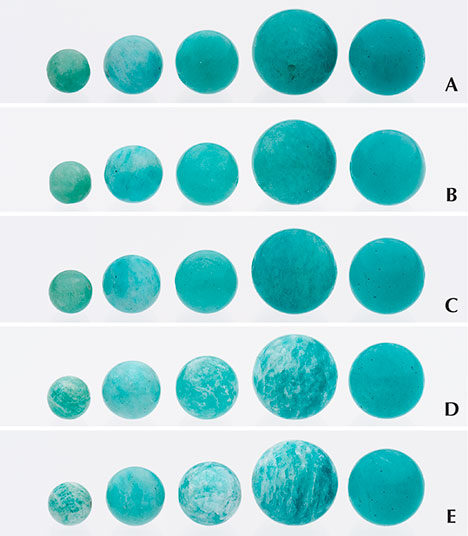
To test their chemical stability, the five beads were immersed in water, reagent alcohol, and then acetone for an hour apiece (figure 3, B–D). The quality of the beads increases gradually from left to right in figure 3. Neither water nor reagent alcohol affected the beads’ appearance (figure 3, B–C). Many whitish fractures became evident on the surface of the smallest, third-largest, and largest beads after immersion in acetone for an hour (figure 3D). Their transparency diminished significantly, and they took on an unsightly mottled appearance. In contrast, the second-smallest and second-largest beads seemed largely unaffected. After 24 hours of immersion in acetone, more whitish fractures became visible in the smallest, third-largest, and largest beads, revealing the true quality of the original material used for impregnation (figure 3E). Very minor whitish fractures were observed in the highest-quality bead (figure 3E, far right). During normal wear, consumers should avoid exposure to solvents that contain large portions of acetone, such as nail polish remover.
.jpg)


