Mixed-Type HPHT Synthetic Diamond with Unusual Growth Features

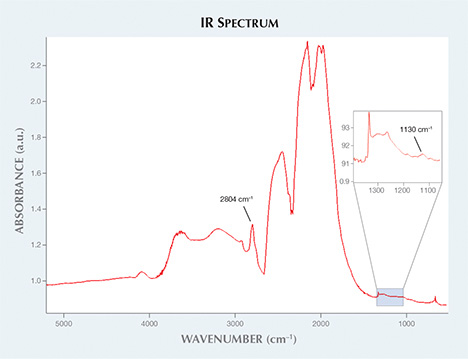
Figure 2. The HPHT-grown synthetic diamond’s infrared spectrum shows peaks related
to both boron and single nitrogen.
to both boron and single nitrogen.

Figure 3. This DiamondView image shows the characteristic growth pattern of an HPHT synthetic diamond. Image by Troy Ardon.
Unless special precautions are taken, diamond synthesis will incorporate nitrogen into the lattice, which leads to a yellow bodycolor. Certain chemicals can be added to prevent the incorporation of nitrogen, and this can lead to colorless diamonds. If boron is introduced to the growth of synthetic diamonds, it will become incorporated and produce a blue bodycolor. If both boron and nitrogen are allowed to incorporate into the lattice, the blue and yellow bodycolors will combine to produce a green color (J.E. Shigley et al., “Lab-grown colored diamonds from Chatham Created Gems,” Summer 2004 G&G, pp. 128–145). Since boron is an electron acceptor and nitrogen is an electron donor, an excess of boron will compensate the nitrogen and the blue color will predominate. Because nitrogen and boron have slight preferences for different growth sectors, if the concentration of both nitrogen and boron is carefully controlled, nitrogen and boron will dominate different growth sectors. The colors will blend to create a face-up greenish color (R.C. Burns et al., “Growth-sector dependence of optical features in large synthetic diamonds,” Journal of Crystal Growth, Vol. 104, No. 2, 1990, pp. 257–279).The growth tubes in this sample were an unusual feature, last seen in a selection of HPHT-grown synthetics several years ago at GIA’s West Coast laboratory. They appear similar to synthetic moissanite growth tubes, though there are a few key differences. Whereas the growth tubes occur in only one direction in synthetic moissanite, they could be seen growing in different directions in this synthetic diamond. The tubes in synthetic moissanite also tend to be very straight, while the ones in this sample curved and changed direction. Although this stone bore some superficial resemblance to moissanite, its gemological properties were quite different, another reminder to avoid quick sight identifications.



